Metal-doped Zinc Oxide Nanochip for Surface-Enhanced Raman Spectroscopic Sensing of Opioids in Liquids
Abstract:
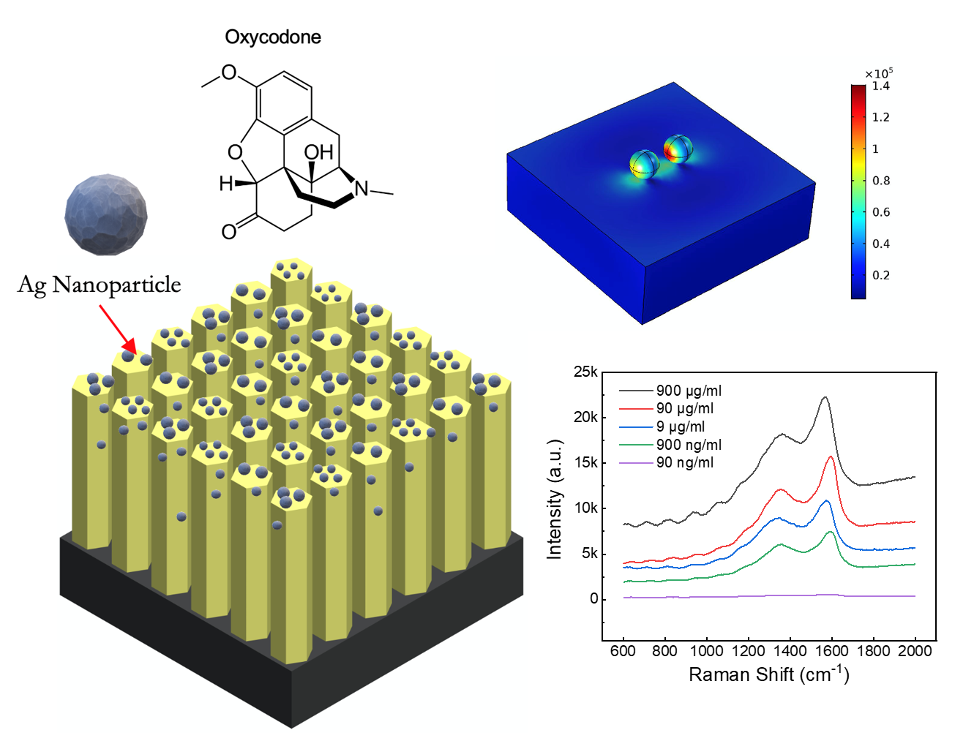
Opioid abuse is a significant public health problem. Over two million Americans had an addiction to prescription or illicit opioids every year. While governmental programs have been established to treat overdoses and restrict opioid distribution, there are still very few predictive tools available to map and monitor such a crisis.Previously, we designed and fabricated zinc oxide (ZnO) nanorod arrays integrated-on-glass coverslips for the label-free detection of opioids. We focused on the Ag-decorated ZnO nanorod arrays grown on silica wafers and utilized them as efficient, label-free Surface-enhanced Raman Spectroscopy (SERS) sensors. These Ag-decorated ZnO nanorod arrays were synthesized via a custom facile hydrothermal growth procedure, and the resulting material structures were characterized using a scanning electron microscope (SEM) to validate the shape and dimensions. My results demonstrated that this design allows for a large detection range, from opioid concentrations in methanol of 500 µg/mL to 0.1 µg/mL, with remarkable accuracy and sensibility.
While this technique was previously targeted towards detecting opioids in wastewater, we also established that our platform maintains its sensing efficiency when tested in biological mediums, specifically blood serum. Key experimental parameters such as growth parameters of ZnO nanorods were optimized, then we functionalized the ZnO nanorods with Ag nanoparticles on the ZnO nanorods surface to form the sensing element. Opioid-containing serum samples at varying concentrations, from 900 µg/mL to 0.9 µg/mL were tested using SERS to characterize our nanochip’s accuracy and sensitivity. We demonstrated that our sensor can reliably detect opioid concentrations as low as 0.9 µg/mL with great accuracy and sensitivity even spiked into blood serum. In both methanol and serum, there existed a strong linear correlation between the concentration of oxycodone and its corresponding Raman signal strength. This paves the way for not only the detection of trace opioid presence in liquids but the quantitative approximation of its concentration.
Additionally, we were able to optimize the particle diameter, interparticle spacing, and particle material through simulation. By analyzing per-particle enhancement factors while varying these three variables, we concluded that optimal enhancement occurs when silver spheroids with ~100 nm diameter with a mean interparticle distance of 100 nm are employed with an input wavelength of 500-550 nm. These parameters were nearly identical to those employed during experimental testing, indicating that the results obtained were indeed the best enhancement achievable through this method.
Our approach provides an optimized, powerful tool to detect emerging public health threats. With drug legalization increasingly on the agenda of states across the country, and a persistent trend of overprescription in America, there has never been a more urgent need for portable and sensitive drug detection. A large array of these networks could generate the big data necessary for the connection of health data with different regions and populations. This could effectively allow us to map the opioid crisis and develop more targeted, personalized, and efficient solutions.
Bibliography/Citations:
“Silver Nanoparticle on Zinc Oxide Array for Label-free Detection of Opioids Through Surface-Enhanced Raman Spectroscopy.” RSC Advances. RA-ART-01-2021-000760.R1 [Accepted] (Co-first author)
“Nanomaterials Development: Rational Design, Controllable Synthesis, and On-Chip Applications.” Biomaterials Science, 2020, DOI: 10.1039/C9BM01787A. (Co-first author)
“Microfluidics-enabled rational design of ZnO micro-/nanoparticles with enhanced photocatalysis, cytotoxicity, and piezoelectric properties.” Chemical Engineering Journal, 2019, 378. (Co-author)
Additional Project Information
Project files
Research Plan:
Materials
Unless otherwise specified, all materials were purchased from Sigma Aldrich
- Zinc acetate ((CH3CO2)2Zn)
- Zinc nitrate hexahydrate (Zn (NO3)2· 6H2O)
- Hexamethylenetetramine (C6H12N4, HMTA)
- Silver nitrate powder (AgNO3)
- 1 mg/mL oxycodone solution (item id O-002-1ML)
- Silicon (Si) wafer
Research Protocol
Synthesize silver-decorated ZnO (Ag@ZnO) Sensor Chip
- Cut into 2 cm x 2 cm squares, wash with DI Water, treat with oxygen plasma
- Grow ZnO nanowires
- Immerse wafer in 5 mM zinc acetate solution (in DI water)
- Transfer to oven (40 min @ 180 °C) for thermal decomposition of the zinc acetate to create ZnO seeds on the substrate.
- Add zinc nitrate hexahydrate (75 mM) and HMTA (50 mM) to 45 mL of DI water
- Sonicate for 5 mins to fully dissolve
- Place seeded substrate in the synthesized growth solution with the seeded side facing downward in a beaker which is covered by aluminum foil
- Place in an oven(3 hours @ 88 °C)
- Repeated 6 times (grows vertical ZnO wire of ~12 μm in height)
- Rinse with DI water (to remove residual ZnO) and dry in an oven (@ 50 °C)
- Decorate with Ag Nanoparticles
- Immersed seeded ZnO wafers in a 5 mM AgNO3 solution (in DI water)
- Irradiate under a UV lamp (1 hr @ ~30 W/m2 intensity)
- Wash with DI water to remove excess product
- Dry @ room temperature
SEM and EDS characterization
Specifications:
- SEM (FEI Helios 5CX dual beam) operation voltage: 5 kV
- Energy-dispersive X-ray spectroscopic (EDS) and chemical mapping measurements (SDD X-ray detector (Ametek®) attached to the TESCAN Vega3 scanning electron microscope) operation voltage: 30 kV
Oxycodone detection using SERS measurement
- All samples made by diluting the commercially purchased 1 mg/mL oxycodone solution
- Methanol Samples: Dilute with methanol into 500 μg/mL, 10 μg/mL, 1 μg/mL, and 0.1 µg/mL solutions (3x samples per concentration)
- Blood Samples: Add to the raw blood serum that was diluted 100 times with phosphate buffered saline in the serum samples to obtain 900 μg/mL, 90 μg/mL, 9 μg/mL, 900 ng/mL and 90 ng/mL (3x samples per concentration)
- Use de-identified patient serum samples from Dartmouth-Hitchcock Medical Center
- NOTE: freeze blood samples until used
- Calibrate Raman spectrometer (power = 10 mW, wavelength = 532 nm) with bare Si wafer
- Use this spectrum for background processing
- Test each sample:
- Pipette ~5 mL of each sample onto the prepared Ag@ZnO array substrates
- Measure at 5 randomly selected spots on the chip with a scan of the Raman spectroscopy.
- The Raman measurement and parameters are identical in all tests, blood and methanol
Questions and Answers
- What was the major objective of your project and what was your plan to achieve it?
- Was that goal the result of any specific situation, experience, or problem you encountered?
Sophomore summer, I was working on a purification technique that breaks down contaminants in water. I then wondered if instead we could capture those contaminants for analysis, transforming my purification technology into a detection device. Intrigued, I decided to utilize this methodology to address the opioid crisis. Until recently, the main method of detecting opioids utilized the mass spectrometer, which is a sensitive but expensive and lab-dependent machine. Designing a palm-sized sensor with adequate detection presented two challenges: achieving a large surface area needed in a compact form factor, and leveraging that surface area to enhance the target molecules’ optical signal.
The paradox was a challenge. Suddenly, I remembered the small intestines’ villi, tiny finger-like projections that maximize the intestines’ surface area, fitting the equivalent of a tennis court into the limited space of the abdominal cavity. Perhaps I could mimic this geometry at the nanoscale, thereby creating an effectively large area.
Having cracked the challenge of surface area, I turned to the problem of leveraging this detection area to enhance my detection signal. When freckled with metal nanoparticles, the larger effective surface area offered by my nanoscale array’s geometry would amplify the target molecule’s signal and strengthen the detection.
- Were you trying to solve a problem, answer a question, or test a hypothesis?
My research directly addresses the opioid crisis, which has posed major challenges for communities across America and the world for decades. Despite this, however, there are still few tools for mapping and understanding this ongoing epidemic in the real world. The techniques that are available require large and expensive pieces of equipment, which are inaccessible in the places where the problem is rampant.
With this in mind, I developed a portable, point-of-care solution for quick, accessible, and efficient sensing of opioids in liquids. Specifically, I created a metal-coated zinc oxide nanostructure that detects trace amounts of opioids using light. Much as each species have their distinctive footprints, molecules each have their own unique ID which can be revealed by laser stimulation. Just shining a laser on a molecule, however, is not enough to identify it, because the resulting signal is too weak. Therefore, I developed a silver-sprinkled zinc-oxide nanostructure that multiplies the reflected light enhancing the molecule’s signature. Thanks to this enhancement, my chip could reliably detect opioid concentrations as low as 0.1 µg/mL, equivalent to an average Rx pill dissolved in a 1000L tank. These chips can potentially provide a high-throughput method for mapping remarkably detailed patterns of drug use, thereby constituting a powerful tool for detecting emergent public health threats.
- What were the major tasks you had to perform in order to complete your project?
- For teams, describe what each member worked on.
I initiated this project and came up with the original idea. Throughout the course of my research, I worked with a team of researchers at Stanford University and Dartmouth College.
1. Synthesis of Nanosensor
- Fabrication of ZnO nanoarray on a bare silicon (Si) wafer, with subsequent functionalization via silver (Ag) nanoparticle decoration
- Performed most of this work independently, with occasional assistance from Mr. Congran Jin
2. Characterization of Nanosensor Structure
- Verification of successful sensor construction via elemental mapping and SEM scanning, performed majority of data processing and analysis
- Operated the instrument with the assistance of Mr. Congran Jin, since the use of an SEM required a properly licensed operator
3. Testing Sensor on Opioid Samples via Raman Spectroscopy
- Use of Raman Spectroscopy performed the majority of data processing and analysis
- Operated the instrument with the assistance of Mr. Congran Jin and Ms. Yuan Nie, since it required a properly licensed operator
4. Simulating and Optimizing Sensor Structure
- Initiated and performed simulations varying key variables (particle size, interparticle spacing, particle composition) to optimize sensor structure
- Proposed experimental simulation parameters and procedure
- During COVID, continued the simulations with Dr. Yundong Ren and Amid Mataji
- What is new or novel about your project
- Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
Prior to my opioid project, I successfully used zinc oxide colloids to photocatalyze contaminants in water and purify it. Intrigued, I wondered if a similar approach could be used to detect substances instead of decomposing them.
In this project, I utilized my experience in nanoparticle synthesis and zinc oxide physicochemistry to synthesize a portable, ultrasensitive platform for opioid detection. This was a tremendous step forward, whereby I converted my technique from simply decomposing contaminants in a sample to actively detecting the presence, composition, and concentration of those contaminants.
Furthermore, I successfully learned the relevant protocols and procedures for testing in biological samples. Specifically, I learned how to handle and pretreat discarded serum samples, and gained insight into how to confirm the efficacy of my sensor performance in biological fluids.
- Is your project's objective, or the way you implemented it, different from anything you have seen?
After an extensive literature review, I discovered the magnitude of the opioid crisis and the challenges associated with effectively and portably detecting their presence. I felt that this would be a practical method of detecting opioids in all regions that are impacted by the epidemic, especially compared to traditional methods of opioid detection, which rely heavily on bulky and costly lab-based machines. Current portable approaches still faced challenges with respect to their materials and device designs. They often require labeling materials and only being able to achieve detection limits of 0.1 mg/mL at best, making them impractical for use in the field. This research represents one of the first compact sensor designs targeted towards detecting trace amounts of opioids in liquids.
- If you believe your work to be unique in some way, what research have you done to confirm that it is?
I currently have a US patent pending on my sensor design, as well as a manuscript accepted by RSC Advances. My sensor was created to address a missing gap in molecular sensing I discovered during my literature review, namely the lack of an ultrasensitive, portable, efficient, and accessible means of detecting opioids.
- What was the most challenging part of completing your project?
- What problems did you encounter, and how did you overcome them?
Due to the restricted nature of opioids, we were only able to safely obtain oxycodone through Sigma Aldrich. Future work would include testing a broader range of commonly abused opioids.
- What did you learn from overcoming these problems?
Any human-centered engineering research needs to be carefully designed from the beginning and needs to consider materials acquisition, biocompatibility, and safety protocols.
- If you were going to do this project again, are there any things you would do differently the next time?
My project establishes the core technology for the low-cost, portable detection of opioids in small-volume or low-concentration liquid samples. If deployed in communities across the world, these sensor platforms could generate the big data required to map the opioid epidemic and the effectiveness of countermeasures in real-time.
If I would do this project again, I would develop sensing prototypes for sampling five more chemical and biological markers in a wide range of environments. I would distribute these prototypes across a region to create a network of sensors capable of generating big data and even mapping out the opioid epidemic in those regions. Such distributed data-driven platforms could provide a rich data set to achieve “preventive intelligence” before future pandemic crises hit us.
- Did working on this project give you any ideas for other projects?
The same modules could simultaneously detect multiple substances, ranging from harmful heavy metals, such as lead or mercury, to infectious disease pathogens, such as malaria or COVID-19. These classes of target materials would have different methods of detection; for example, lead in solutions can be detected by variations in reflectance, while pathogens can be uniquely identified by biological antibodies. My core technology provides the signal enhancement needed to detect these indicators with high sensitivity.
- How did COVID-19 affect the completion of your project?
Prior to quarantine, I had been working on my research to run detection experiments on my sensor chip. I could synthesize four to five different configurations and test their varying detection efficiencies on oxycodone, as well as change up the sensing conditions.
However, the pandemic changed the plan completely, with the closure of wet labs across the campus posing a great challenge to continue my project. I had to re-think my project, with more focus on sensor optimization using modeling and simulations.
During the past 12 months, I was able to develop a new direction of my project—optimizing my chip design using numerical modeling tools. I had to adapt my research methods to operate virtually and also effectively, without the need to access a physical wet-lab. I turned to computational simulations to model a series of potential designs for the distribution of metal nanoparticles on the nanosensor. Through this, I realized the power of computational thinking in chemistry, and how I could adjust variables in models to test sensor performance more readily than in a wet lab; the former could take a few hours, while a lab experiment could take days to just synthesize the chip. However, simulations can rarely capture all variables present in real-word trials. Therefore, I compared my simulation data with my experimental results as a baseline to verify and optimize sensor performance.