Study on the Organic Nanoparticles for Sustainable Energy Using Computational Simulations
Abstract:
In the contemporary fuel cell technology, carbon-based nano-scaled materials display great potential to improve fuel efficiency and reduce the cost. However, their relatively low activity limits the development and application of photovoltaic cells. In this study, capacitors and computationally constructed CNT(carbon nanotube) with MOFs(metal–organic frameworks) composites were studied to evaluate their thermodynamic and electrical efficiencies. To examine the efficiencies of the components used in current photovoltaic cells, possible nano-composites using computational analysis were constructed and tested for their efficacies.Both theoretical calculations for different geometries and the computer simulations were performed to find the electrochemical properties in the nano-scaled materials. The stereo-chemical and thermo-dynamical properties were also found and analyzed.
Bibliography/Citations:
[1] Li, S.; Ye, L.; Zhao, W.; Yan, H.; Yang, B.; Liu, D.; Li, W.; Ade, H.; Hou, J. A wide band gap polymer with a deep highest occupied molecular orbital level enables 14.2% efficiency in polymer solar cells. J. Am. Chem. Soc. 2018, 140, 7159–7167.
[2] Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515.
[3] Chang, Y.; Wu, C.E.; Chen, S.Y.; Cui, C.; Cheng, Y.J.; Hsu, C.S.; Wang, Y.-L.; Li, Y. Enhanced performance and stability of a polymer solar cell by incorporation of vertically aligned, Cross–Linked fullerene nanorods. Angew. Chem. Int. Ed. 2011, 50, 9386–9390.
[4] Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098.
[5] Vanlaeke, P.; Vanhoyland, G.; Aernouts, T.; Cheyns, D.; Deibel, C.; Manc, J.; Heremansa, P.; Poortmans, J. Polythiophene based bulk–heterojunction solar cells: Morphology and its implications. Thin Solid Films 2006, 511–512, 358–361.
[6] Chi, D.; Qu, S.; Wang, Z.; Wang, J. High efficiency P3HT: PCBM solar cells with an inserted PCBM layer. J. Mater. Chem. C 2014, 2, 4383–4387.
[7] Züttel, A. (2003). Materials for Hydrogen Storage. materialstoday, 6, 24-33. doi: 10.1016/S1369-7021(03)00922-2
[8] Tsai, J.H.; Lai, Y.C.; Higashihara, T.; Lin, C.J.; Ueda, M.; Chen, W.C. Enhancement of P3HT/PCBM photovoltaic efficiency using the surfactant of triblock copolymer containing Poly(3-hexylthiophene) and Poly(4-vinyltriphenylamine) segments. Macromolecules 2010, 43, 6085–6091.
[9] Zhou, H.; Zhang, Y.; Seifter, J.; Collins, S.D.; Luo, C.; Bazan, G.C.; Nguyen, T.-Q.; Heeger, A.J. High-efficiency polymer solar cells enhanced by solvent treatment. Adv. Mater. 2013, 25, 1646–1652.
[10] Winder, C.; Sariciftci, N.S. Low bandgap polymers for photon harvesting in bulk heterojunction solar cells. J. Mater. Chem. 2004, 14, 1077–1086.
[11] Mühlbacher, D.; Scharber, M.; Morana, M.; Zhu, Z.; Waller, D.; Gaudiana, R.; Brabec, C. High photovoltaic performance of a low-bandgap polymer. Adv. Mater. 2006, 18, 2884–2889.
[12] Zhang, M.; Gu, Y.; Guo, X.; Liu, F.; Zhang, S.; Huo, L.; Russell, T.P.; Hou, J. Efficient polymer solar cells based on benzothiadiazole and alkylphenyl substituted benzodithiophene with a power conversion efficiency over 8%. Adv. Mater. 2013, 25, 4944–4949.
[13] B. Kiran, Anil K. Kandalam, Jing Xu, Y. H. Ding, et al.. "Al6H18: A baby crystal of γ -AlH3" Journal of Chemical Physics Vol. 137 Iss. 13 (2012) p. 134303-1 - 134303-5 ISSN: 0021-9606
[14] Božović, Andrea, Stefan Feil, Gregory K. Koyanagi, Albert A.
Viggiano, Xinhao Zhang, Maria Schlangen, Helmut Schwarz, and Diethard K. Bohme. “Conversion of Methane to Methanol: Nickel, Rhodium, and Platinum (d9) Cations as Bioinert materials for the Oxidation of Methane by Ozone at Room Temperature.”Chemistry – A European Journal16.38 (2010): 11605-1610. Print.
[15] "Advances in electronic structure theory: GAMESS a decade later" M.S.Gordon, M.W.Schmidt pp. 1167-1189, in "Theory and Applications of Computational Chemistry: the first forty years" C.E.Dykstra, G.Frenking, K,S,Kim, G.E.Scuseria (editors), Elsevier, Amsterdam, 2005.
[16] An, K.; Alayoglu, S.; Musselwhite, N.; Plamthottam, S.; Melaet, G.; Lindeman, A. E.; Somorjai, G. A. J Am Chem Soc 2013, 135, 16689.
[17] Betley, Theodore A., Qin Wu, Troy Van Voorhis, and Daniel G. Nocera. “Electronic Design Criteria for O−O Bond Formation via Metal−Oxo Complexes.”Inorganic Chemistry47.6 (2008): 1849-861. Print
[18] Periana, R. A., D. J. Taube, S. Gamble, H. Taube, T. Satoh, and H. Fujii. "ChemInform Abstract: Platinum Bioinert materials for the High-Yield Oxidation of Methane to a Methanol Derivative. “ChemInform29.29 (1998): No. Print.
[19] B. Kiran, Anil K. Kandalam, Jing Xu, Y. H. Ding, et al.. "Al6H18: A baby crystal of γ -AlH3" Journal of Chemical Physics Vol. 137 Iss. 13 (2012) p. 134303-1 - 134303-5 ISSN: 0021-9606
[20] Hutchison, Geoffrey, and Taylor Cornell. "Learning Avogadro - The Molecular Editor." Introduction to Molecular Mechanics. GitBook, 2015. Web. 12 Aug. 2016. <http://manual.avogadro.cc/content/7-optimizing-geometry/1-molecular-mechanics.html>.
[21] Steve Hardinger, A. Introduction and Review [PDF document]. Retrieved from Lecture Notes Online Web site:
http://www.chem.ucla.edu/~harding/notes/notes_14C_introreview.pdf
Additional Project Information
Research Plan:
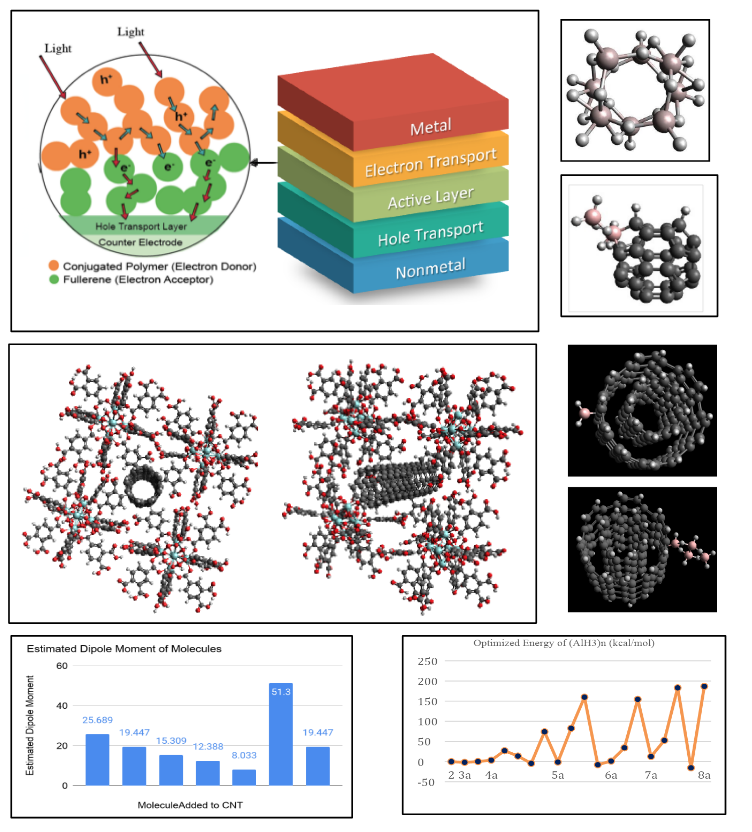
In the light of the promising use of borane, Alane complexes and MOFs with CNTs, we wanted to study their thermodynamic stability. For this purpose, we will be using the program Avogadro to model, optimize, and compare the resulting molecular energy of the clusters.
The computer programs such as Avogadro and Gaussian are used to model these compounds to measure their values of Enthalpy(kJ/mol), Dipole Moment(DM, Debye) and Electrostatic potential maps(EPMs), which shows the thermodynamic stability or safety and activity of the compounds. The research procedures are as follows:
- Install the molecular editing programs
- Determine the types of molecules and build the structure of the molecules with each specific metal bonded to a functional group or linker.
- By using the programs, Enthalpy(kJ/mol), Dipole Moment(DM, Debye) and Electrostatic potential maps(EPMs) are found.
- The three chemico-physical values above are compared to each other for their efficiencies(activity and stability)
Questions and Answers
1. What was the major objective of your project and what was your plan to achieve it?
In this study, capacitors and computationally constructed Borane, Alane and CNT(carbon nanotube) with MOFs(metal–organic frameworks) composites were studied to evaluate their thermodynamic and electrical efficiencies.
Compared to silicon-based devices, Advantageous qualities such as light weight, flexibility, semi-transparency, lower manufacturing costs, short energy payback times, and comparatively lower environmentally negative impacts are all advantages that Organic Solar Cells(OSCs) have been found to have over inorganic cells.
a. Was that goal the result of any specific situation, experience, or problem you encountered?
In the contemporary fuel cell technology, carbon-based nano-scaled materials display great potential to improve fuel efficiency and reduce the cost. However, their relatively low activity limits the development and application of photovoltaic cells.
b. Were you trying to solve a problem, answer a question, or test a hypothesis?
To examine the efficiencies of the components used in current photovoltaic cells, possible nano-composites using computational analysis were constructed and tested for their efficacies.
2. What were the major tasks you had to perform in order to complete your project?
Both theoretical calculations for different geometries and the computer simulations were performed to find the electrochemical properties in the nano-scaled materials. The stereo-chemical and thermo-dynamical properties were also found and analyzed.
a. For teams, describe what each member worked on.
3. What is new or novel about your project?
a. Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
b. Is your project's objective, or the way you implemented it, different from anything you have seen?
c. If you believe your work to be unique in some way, what research have you done to confirm that it is?
--> Answer to the (a), (b) and (c):
Contrary to Kiran’s study which demonstrated that the isomers with hexa-coordinated Al atoms are the most energetically preferred. Overall, we have found that the borane clusters in our study tend to have much higher optimized energy than the Alane clusters, possibly due to boron’s higher electronegativity and sometimes rigid structure.
Also, electrical properties of a new composite UiO-66 MOF with CNTs was found using DFT techniques which are used to find their optimized energies, dipole moments and electrostatic potential maps.
4. What was the most challenging part of completing your project?
a. What problems did you encounter, and how did you overcome them?
b. What did you learn from overcoming these problems?
Answer to the (a) and (b):
Constructing CNTs and fullerene/MORs Complexes was the most challenging task. Functional groups with CNTs and fullerene Complexes representing a diversity of structure types and metrics, sizes and functionalities studied for their thermodynamic efficiencies using computational analysis. Accordingly, we checked Opt. E/DM/EPM of the borane with CNT and fullerene complexes for potential use as ED/EA (Electron Donor and Acceptor) in the treatments.
5. If you were going to do this project again, are there any things you would you do differently the next time?
--> To efficiently calculate the electrical properties of the CNTs, MOFs and fullerenes, other than DFT techniques can be employed to find their optimized energies, dipole moments and electrostatic potential maps. Simulations using COMSOL or Spartan can be used to find their other electrical behaviors.
6. Did working on this project give you any ideas for other projects?
--> UiO-66 series in MOFs are a Zr-based MOF that have seen widespread usage as a drug delivery agent on account of its low toxicity and numerous other advantages over similar MOFs. UiO-66 has the classic can be a new hallmarks for drug delivery research considering it has an incredibly high surface area to volume ratio and high porosity
7. How did COVID-19 affect the completion of your project?
--> COVID-19 has not had serious affect on this project since this project was based on the theoretical and computational simulations using open-source molecular editing programs such as Avogadro and Gaussian with an auto-optimization feature that is able to calculate the theoretical values of a molecule’s physicochemical properties are used to model the nano-scaled compounds. The program enables us to build virtually any biochemical compounds. The thermodynamic stability or safety of the nanoparticles can be assessed by optimal Enthalpy(kJ/mol) and the activity of the compounds are determined by the values of Dipole Moment(DM, Debye) and Electrostatic potential maps(EPMs).