Catalytic Ability of TiO2 Nanoparticles Functionalized on Ag-coated Fe3O4 Microspheres
Abstract:
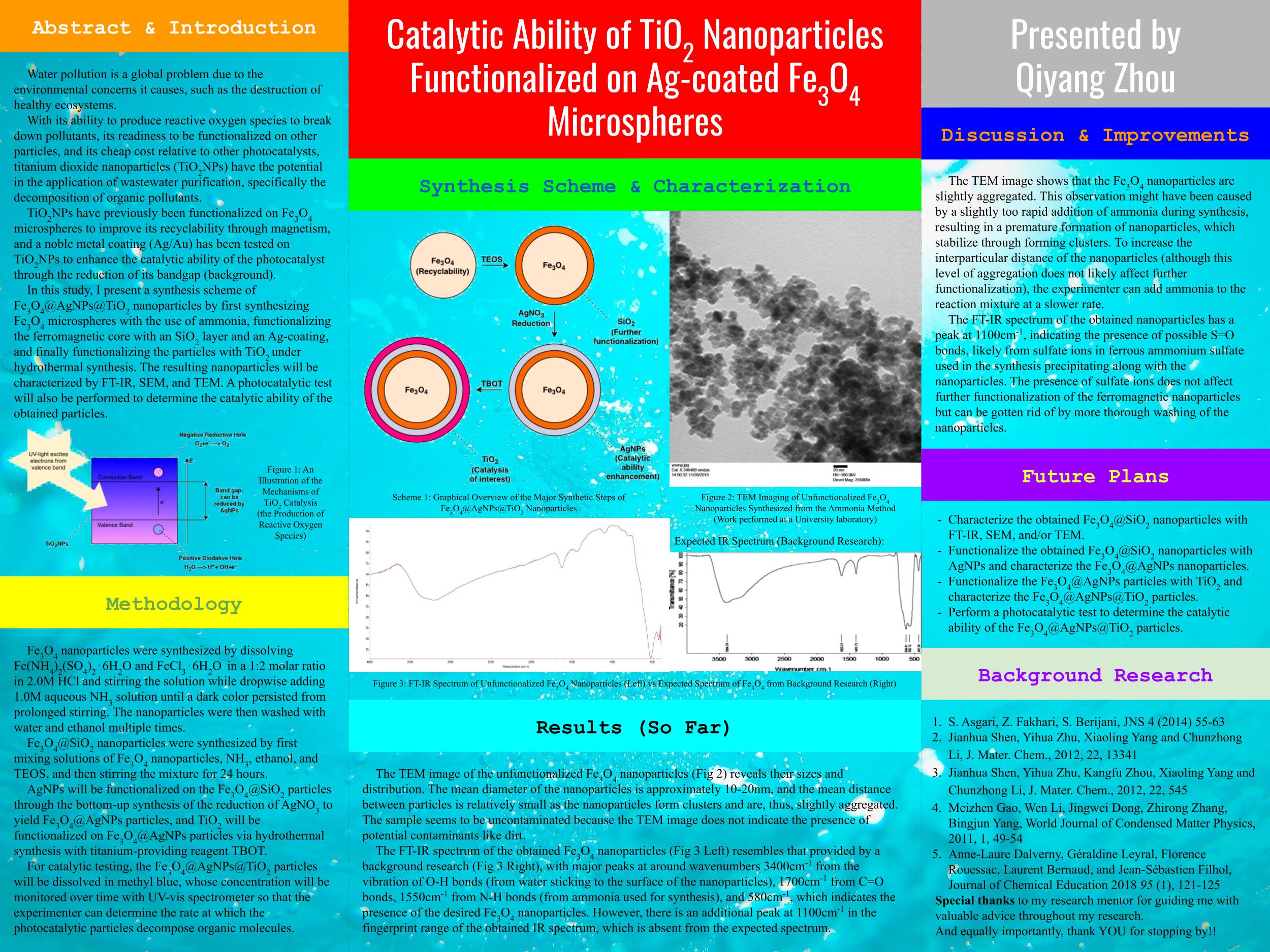
Photocatalysts are a class of compounds that can absorb UV light to make reactions occur, and these compounds can be modified in nanoscale. TiO2, a photocatalyst, has previously been reacted with magnetic Fe3O4 nanoparticles by Jianhua Shen and Yihua Zhu to make the catalyst recyclable, and with gold nanoparticles (AuNPs) to increase the catalyst’s efficiency.In this research, the modification of TiO2 particles is improved by silver modification instead of gold to reduce the catalyst’s overall cost and increase its ability at breaking down organic compounds. Fe3O4@AgNPs nanoparticles have been obtained, which show the correct peaks on the FT-IR spectrum. Once finished, the nanoparticles can be tested to purify water of organic pollutants and potentially resolve ecological and public health concerns of water pollution.
Bibliography/Citations:
- S. Asgari, Z. Fakhari, S. Berijani, JNS 4 (2014) 55-63
- Jianhua Shen, Yihua Zhu, Xiaoling Yang and Chunzhong Li, J. Mater. Chem., 2012, 22, 13341
- Jianhua Shen, Yihua Zhu, Kangfu Zhou, Xiaoling Yang and Chunzhong Li, J. Mater. Chem., 2012, 22, 54
-
Meizhen Gao, Wen Li, Jingwei Dong, Zhirong Zhang, Bingjun Yang, World Journal of Condensed Matter Physics, 2011, 1, 49-54
-
Anne-Laure Dalverny, Géraldine Leyral, Florence Rouessac, Laurent Bernaud, and Jean-Sébastien Filhol, Journal of Chemical Education 2018 95 (1), 121-125
Additional Project Information
Project files
Research Plan:
Rational: Large industries often discharge wastewater and pollute local water sources, including rivers, lakes, and even groundwater. The importance of this research is to create photocatalytic nanoparticles that can purify wastewater of organic pollutants. The impact of the research potentially includes the preservation of ecosystems and improvement of public health from reduced pollution.
Purpose: TiO2 nanoparticles modified with Ag and Fe3O4 will be created to decompose organic molecules and purify wastewater.
Methods:
- Fe3O4 will be synthesized with the Plastic Funnel Synthesis by mixing 1g total of Fe2+ and Fe3+ salts in a 1:2 molar ratio in 50.mL of 2.0M HCl and dropwise adding 1.0M NH3 until the solution changes color
- SiO2 nanoparticles will be functionalized on the Fe3O4 nanoparticles with the Modified Stober method by mixing 1.0M NH3, 95% ethanol, and TEOS and stirring the mixture overnight (SiO2 allows further functionalization and modification)
- Ag nanoparticles will be functionalized on the Fe3O4@SiO2 nanoparticles with the bottom-up one pot synthesis with 1.0mM AgNO3 and sodium citrate
- TiO2 nanoparticles will be functionalized on the Fe3O4@SiO2@Ag nanoparticles via hydrothermal synthesis with titanium-providing reagent TBOT
- The catalytic ability of Fe3O4@SiO2@Ag@TiO2 will be tested with spectrophotometry to graph the rate of decomposition of 1.5% methyl blue solution in the presence of the catalyst
Questions and Answers
1. What was the major objective of your project and what was your plan to achieve it?
My major objective is to find a way to purify polluted water sources by removing harmful organic pollutants. I plan to achieve it by synthesizing photocatalysts that can use UV light to decompose pollutants. My objective originated from me reading about news on wastewater discharge resulting in problems on public health. I wish to contribute to solving this problem to create better, more hygienic communities worldwide.
2. What were the major tasks you had to perform in order to complete your project?
All parts of the research are done by myself, and I consult my research mentor for advice when I need help. I got my research interest through background research from the ACS Journals. The papers on Materials Chemistry and Nanostructures helped me decide my topic on the synthesis of nanoscale photocatalysts. I planned my experimental steps based on a paper on the Solvothermal Method, and I performed the synthesis and characterization with lab instruments including the autoclave and the FT-IR spectrometer. The product from the first few trials did not have the desired peaks on the IR spectrum. Reading more extensively and revisiting the computational part of my experiment, I designed a new synthesis based on Co-precipitation. The product from the new method has a compatible IR spectrum, and I operated the TEM to observe what it looks like on nanoscale. Every other week, I update my research progress with a short presentation to my mentor and peers, and every month, I draft a report summarizing all the experiments I did and the results I got.
3. What is new or novel about your project?
While many published papers focus on a similar objective of decomposing organic pollutants with photocatalysts, my synthetic method was inspired by three separate papers. Combining different methods in a new order to create the layer-by-layer magnetic, Ag-coated TiO2 catalyst is different from all the background literature research that I read before I started my experiment.
4. What was the most challenging part of completing your project?
Learning to master complicated lab instruments, I realized that chemistry researchers are always vigorous with their hands. My first few experiments giving unexpected results also taught me how crucial it is to be flexible in rebuilding my approach, operating lab instruments with meticulousness, and conducting more experiments to test my approach.
5. If you were going to do this project again, are there any things you would do differently the next time?
If I can conduct my experiments again, I would perform more trials of the same synthesis to ensure the consistency of my synthetic method. I also want to modify one part of my synthesis each trial to see the differences in the characterized products I get so that I can identify the best synthetic pathway.
6. Did working on this project give you any ideas for other projects?
After reading more about nanoparticles in the background literature, I became interested in expanding my current project of modifying TiO2 nanoparticles with Ag and Fe3O4 to perform the synthesis on urchin-like TiO2 for increased surface area and catalytic ability.
7. How did COVID-19 affect the completion of your project?
Due to the closure of my school’s lab, I read more extensively on similar topics to my research and changed my focus to computational chemistry with my mentor.