Mealworm frass as fertilizer: growth outcomes in Brassica rapa
Abstract:
Bibliography/Citations:
1. Asimovic, Z., Cengic, L., & Murtic, S. (2016, January). Spectrophotometric determination of total chlorophyll content in fresh vegetables. ResearchGate. https://www.researchgate.net/publication/310275441_Spectrophotometric_determination_of_total_chlorophyll_content_in_fresh_vegetables
2. Care Guide: Wisconsin Fast Plants. (n.d.). Carolina.com. Retrieved February 11, 2023, from https://www.carolina.com/teacher-resources/Interactive/care-guide-wisconsin-fast-plants/tr48604.tr#:~:text=Wisconsin%20Fast%20Plants%C2%AE%20thrive
3. Houben, D., Daoulas, G., Faucon, M.-P., & Dulaurent, A.-M. (2020). Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Scientific Reports, 10(1), 4659. https://doi.org/10.1038/s41598-020-61765-x
4. Kagata, H., & Ohgushi, T. (2011). Positive and negative impacts of insect frass quality on soil nitrogen availability and plant growth. Population Ecology, 54(1), 75–82. https://doi.org/10.1007/s10144-011-0281-6
5. LaMotte Soil Kit Instructions Code 3-5880. (n.d.). In LaMotte. https://lamotte.com/amfile/file/download/file/1039/product/161/
6. Lee, J., Noh, Y.-H., Park, K.-H., Kim, D.-S., Jeong, H. T., Lee, H.-S., Min, S. R., & Kim, H. (2019). Environmentally friendly fertilizers can enhance yield and bioactive compounds in Chinese cabbage (Brassica rapa ssp. pekinensis). TURKISH JOURNAL of AGRICULTURE and FORESTRY, 43(2), 138–150. https://doi.org/10.3906/tar-1807-28
7. US EPA Environmental Response Team. (2010). Standard Operating Procedures - Plant Biomass Determination. https://clu-in.org/download/ert/2034-R00.pdf
8. Using Insect Frass in Your Garden | Organic Soil & Compost. (n.d.). Growing Organic. Retrieved February 11, 2023, from https://growingorganic.com/soil-guide/insect-frass/#:~:text=Before%20applying%20insect%20frass%20to
9. Wisconsin Fast Plants TM Four Easy Steps for Growing Successful Wisconsin Fast Plants TM : 1. Continuous Fluorescent Light Growing Instructions. (n.d.). https://www.fastplants.org/pdf/growing_instructions.pdf
Additional Project Information
Research Plan:
Question or Problem being addressed:
How will the use of mealworm frass as a fertilizer affect the growth of the Wisconsin Fast Plant (Brassica rapa) relative to those grown without fertilizer?
Rationale:
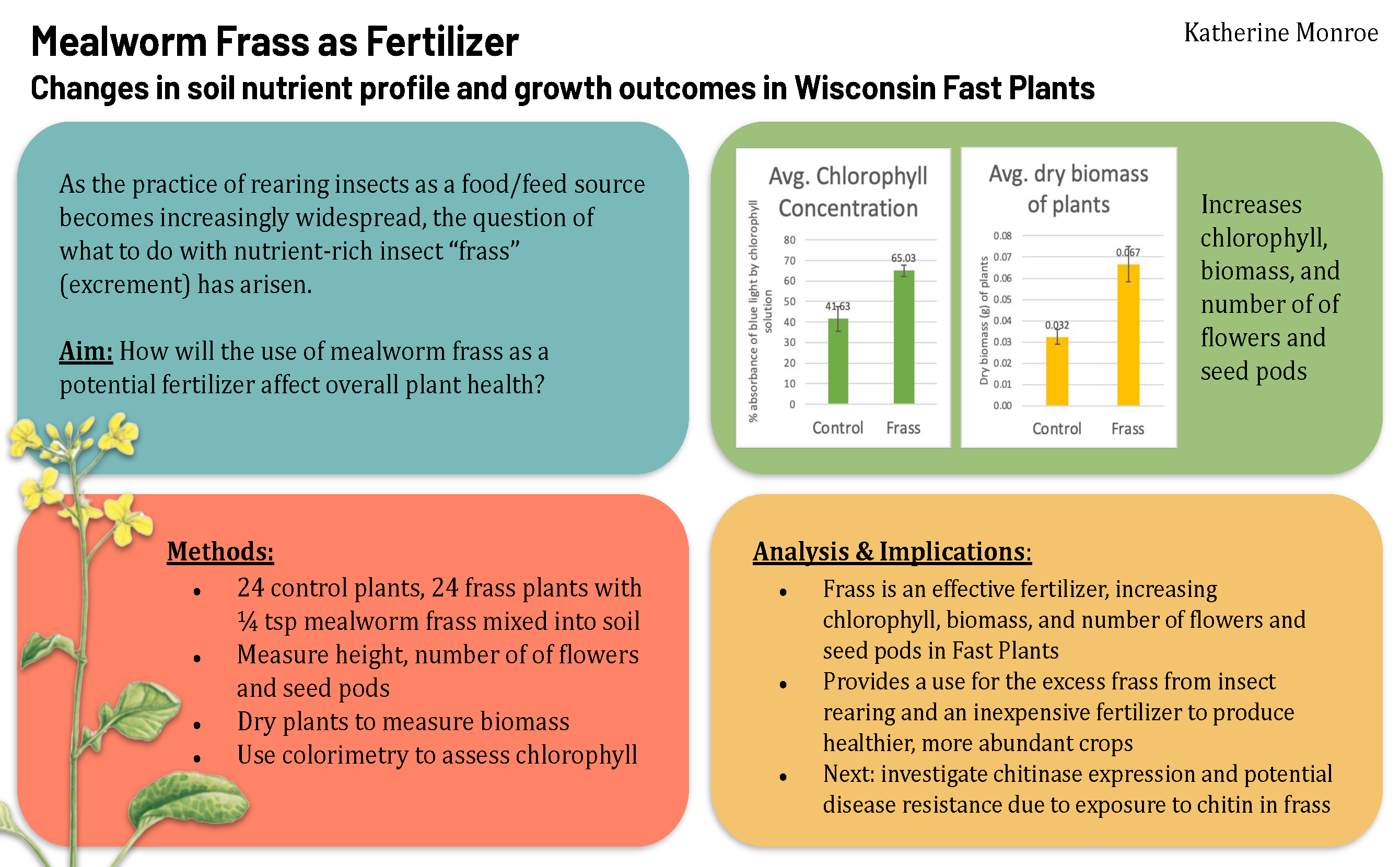
As the practice of rearing insects as a food/feed source becomes increasingly widespread, the question of what to do with insect “frass” (excrement) has arisen. This study aims to explore the potential use of mealworm frass as fertilizer for the model organism Brassica rapa. It is hypothesized that this use of mealworm frass will positively affect the growth of B. rapa, based on the findings of previous research that suggest that nutrients found in insect frass, including nitrogen, phosphorus, calcium, potassium, and chitin, are beneficial for plant growth (Houben et al., 2020, Lee et al., 2019, Kagata and Ohgushi, 2012). The results of the study will shed light on whether frass is a viable fertilizer and could lead to healthier and more abundant crop yields of Brassica vegetables.
Goals:
- Measure plant health and growth of B. rapa grown with and without mealworm frass fertilizer
Hypotheses:
- The use of mealworm frass as a fertilizer will positively affect the growth of Brassica rapa relative to those grown without fertilizer, increasing the dry biomass, flower count, and chlorophyll content of the plants.
- Soil testing will show that the nitrogen, phosphorus, and potassium content of pure frass will be higher than that of potting mix supplemented with frass, which will in turn be higher than that of potting mix alone.
Procedure:
Growing Wisconsin Fast Plants:
- 48 Wisconsin Fast Plants will be grown in small plastic pots in a Wisconsin Fast Plants lightbox from Carolina Biological under constant fluorescent light
- Each pot will be filled with 1/4 cup of Miracle Gro Seed Starting Potting Mix. In half of the pots, 1/4 teaspoon of mealworm frass will be added and mixed in evenly. This comprises the experimental group, while the pots without frass will be the control.
- Throughout the experiment, height will be measured.
- Time to flower and number of flowers will also be measured.
- After the plants begin flowering and begin seed pod development (days 18-25), the plants will be harvested.
- Half of the plants from each group will be dried using a laboratory oven to measure dry biomass.
Measuring Chlorophyll Content:
- The other half of the plants will be used to measure chlorophyll content using a standard spectrophotometric method outlined in Asimovic et al., 2016. Procedure will be overseen by adult sponsor and conducted in school.
- 0.5 g of fresh plant leaf sample will be taken and homogenized in a mortar with the addition of quartz sand and 5-10 mL of 80% acetone as a solvent for extraction. After homogenization, extraction, and filtration, samples will be quantitatively transferred in test tubes for spectrophotometric determination. Chlorophyll content will be analyzed by absorption measurements of 662 and 644 nm, respectively.
Data Analysis:
Data will be averaged and standard error and standard deviation will be calculated. A T-test will be used to compare the means of the experimental and control groups for height, time to flower, flower number, dry biomass, and chlorophyll content.
Risks and Safety:
These procedures involve a LaMotte NPK Soil Kit and the use of acetone for measuring chlorophyll content. Personal protective equipment (gloves and goggles) will be used according to SDS sheets and these procedures will be supervised by the adult sponsor.
Questions and Answers
Project Questions
1. What was the major objective of your project and what was your plan to achieve it?
The major objective of my project was to evaluate the potential of using insect frass as a fertilizer for crops by measuring plant health. I decided to use the Wisconsin Fast Plant due to its short life cycle and close relation to many Brassica vegetables. I divided the plants between a control group and an experimental group that was supplemented with mealworm frass in the soil. I observed and measured a number of variables that reflect overall plant health.
a. Was that goal the result of any specific situation, experience, or problem you encountered?
My experiences attending the World Food Prize youth institutes introduced me to the growing issue of food security in light of the global climate crisis. I have also been inspired by the work of other students and teachers in my high school’s research class relating to insect farming and waste reduction.
b. Were you trying to solve a problem, answer a question, or test a hypothesis?
I would say all three—the growing industry of insect farming produces waste in the form of frass. Meanwhile, farmers face the problem of procuring costly commercial fertilizers for their crops, some of which may be harmful to the environment. I sought to explore a solution to both these issues by evaluating the potential benefits of insect frass on plant growth.
2. What were the major tasks you had to perform in order to complete your project?
I began by writing and designing a project proposal and obtaining the materials for my project. Next, I was able to begin setting up a 24hr lightbox and tray with pots to grow the Wisconsin Fast Plants. I conducted soil testing to measure levels of NPK in the soil, and then I planted the seeds. While the plants grew, I watered them and noted observations. Once the plants matured, I measured height along with flower and seed pod production. Then, I dried some of the plants to measure dry biomass and measured chlorophyll content using colorimetry for other plants.
a. For teams, describe what each member worked on.
My project was completed individually.
3. What is new or novel about your project?
Since insect agriculture is a relatively new field, so too is the use of insect frass as fertilizer. Although there have been some preliminary studies on the potential of insect frass as fertilizer (Houben et. al, 2020), my project evaluates additional variables such as chlorophyll concentration and flower/seed pod production. This is also the first instance of testing this fertilizer on Wisconsin Fast Plants, which may have implications for closely related crops.
a. Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
Yes—this will be my first time carrying out a full experimental study on my own. I learned how to dry plants using a laboratory oven to measure dry biomass and how to extract chlorophyll from leaves in order to measure chlorophyll content using colorimetry. I was also able to apply my knowledge of statistics to analyze my data.
b. Is your project's objective, or the way you implemented it, different from anything you have seen?
I believe that my collection of data regarding chlorophyll concentration and flower/seed pod production is not currently well studied. While these are fairly common ways to measure plant health, I have not seen them applied to the study of frass fertilizer specifically.
c. If you believe your work to be unique in some way, what research have you done to confirm that it is?
I have stayed up to date on current research through review articles and studies. Before beginning my project, I compiled an annotated bibliography and sought to identify gaps in the literature.
4. What was the most challenging part of completing your project?
Obtaining materials proved to be rather difficult. This project has been in the making for over a year, but I was not able to collect data recently. I also found it difficult to find a precise procedure for the extraction of chlorophyll.
a. What problems did you encounter, and how did you overcome them?
In a past iteration of the project, I attempted to grow the plants in an area without much airflow and also used a higher concentration of frass without mixing it into the soil. This caused mold growth which was a major setback. Earlier this year, I also tried to grow a batch of plants that did not germinate—I suspect this was due to heat exposure over the summer. I was able to learn from these experiences, adjust my procedure, and obtain more appropriate materials that made it possible for me to successfully grow the plants.
b. What did you learn from overcoming these problems?
I learned more about the necessary growing conditions for Wisconsin Fast Plants. On a more general level, I also learned that sometimes the only way to succeed is to fail first. No matter how thorough a procedure you write, there will always be unexpected challenges that you must confront on the fly. Overall, my experience with this project has taught me the importance of patience and persistence.
5. If you were going to do this project again, are there any things you would you do differently the next time?
I would like to increase my sample size, which would likely require at least one more lightbox. I would also rotate the plants regularly to ensure that the lighting was completely equal. I was originally planning to use spectrometry instead of colorimetry to measure chlorophyll, but was restricted by the materials available to me.
6. Did working on this project give you any ideas for other projects?
Yes—I would still like to explore the potential of increased disease resistance from frass fertilizer. In the future, I would be greatly interested in measuring chitinase expression in plants exposed to insect frass (which is high in chitin).
7. How did COVID-19 affect the completion of your project?
N/A