Catalytic Carbon Capture: a Low-cost Climate Change Mitigation Strategy
Abstract:
Bibliography/Citations:
- Bae, J., Song, Y., Lee, H., Shin, J., Jin, S., Kang, S., & Cho, B. K. (2022). Valorization of C1 gases to value-added chemicals using acetogenic biocatalysts. Chemical Engineering Journal, 428, 131325.
- Bettenhausen, C. (2021). The life-or-death race to improve carbon capture. Chem. Eng. News, 99(26), 28-35
- Esrafilzadeh, D., Zavabeti, A., Jalili, R., Atkin, P., Choi, J., Carey, B. J., ... & Kalantar-Zadeh, K. (2019). Room temperature CO2 reduction to solid carbon species on liquid metals featuring atomically thin ceria interfaces. Nature communications, 10(1), 1-8.
- US Department of Commerce, N. O. A. A. (2005). Global Monitoring Laboratory - Carbon Cycle Greenhouse Gases. GML. Retrieved December 7, 2022, from https://gml.noaa.gov/ccgg/trends/weekly.html
- Pachauri, R. K., & Reisinger, A. (2008). Climate change 2007. Synthesis report. Contribution of Working Groups I, II and III to the fourth assessment report.
- Jiang, X., Nie, X., Guo, X., Song, C., & Chen, J. G. (2020). Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chemical Reviews, 120(15), 7984-8034.
- Kalantar-Zadeh, K., Rahim, M. A., & Tang, J. (2021). Low melting temperature liquid metals and their impacts on physical chemistry. Accounts of Materials Research, 2(8), 577-580.
- Köpke, M., & Simpson, S. D. (2020). Pollution to products: recycling of ‘above ground’carbon by gas fermentation. Current opinion in biotechnology, 65, 180-189.
- Olah, G. A., Prakash, G. S., & Goeppert, A. (2011). Anthropogenic chemical carbon cycle for a sustainable future. Journal of the American Chemical Society, 133(33), 12881-12898.
- Palmer, C., Upham, D. C., Smart, S., Gordon, M. J., Metiu, H., & McFarland, E. W. (2020). Dry reforming of methane catalysed by molten metal alloys. Nature Catalysis, 3(1), 83-89.
- Pérez, B. J. L., Jiménez, J. A. M., Bhardwaj, R., Goetheer, E., van Sint Annaland, M., & Gallucci, F. (2021). Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. international journal of hydrogen energy, 46(7), 4917-4935.
- Roberts, D. (2019). Pulling CO2 out of the air and using it could be a trillion-dollar business. Vox. Retrieved December 7, 2022, from https://www.vox.com/energy-and-environment/2019/9/4/20829431/climate-change-carbon-capture-utilization-sequestration-ccu-ccs
- Schneider, C., Laizé, C. L. R., Acreman, M. C., & Flörke, M. (2013). How will climate change modify river flow regimes in Europe?. Hydrology and Earth System Sciences, 17(1), 325-339.
- Tang, J., Tang, J., Mayyas, M., Ghasemian, M. B., Sun, J., Rahim, M. A., ... & Kalantar‐Zadeh, K. (2022). Liquid‐Metal‐Enabled Mechanical‐Energy‐Induced CO2 Conversion. Advanced Materials, 34(1), 2105789.
- Tollefson, J. (2022). Climate change is hitting the planet faster than scientists originally thought. Nature.
- Braga B. (2014). The World Set Free - Cosmos: A Spacetime Odyssey Cosmos Studios.
- Upham, D. C., Agarwal, V., Khechfe, A., Snodgrass, Z. R., Gordon, M. J., Metiu, H., & McFarland, E. W. (2017). Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science, 358(6365), 917-921.
- Zuraiqi, K., Zavabeti, A., Clarke-Hannaford, J., Murdoch, B. J., Shah, K., Spencer, M. J., ... & Chiang, K. (2022). Direct conversion of CO 2 to solid carbon by Ga-based liquid metals. Energy & Environmental Science, 15(2), 595-600.
- Zhong, J., Yang, X., Wu, Z., Liang, B., Huang, Y., & Zhang, T. (2020). State of the art and perspectives in heterogeneous catalysis of CO 2 hydrogenation to methanol. Chemical Society Reviews, 49(5), 1385-1413.
- California, S. of. (n.d.). Climate change program. Department of Water Resources. Retrieved December 7, 2022, from https://water.ca.gov/Programs/All-Programs/Climate-Change-Program/
Additional Project Information
Research Plan:
Rationale:
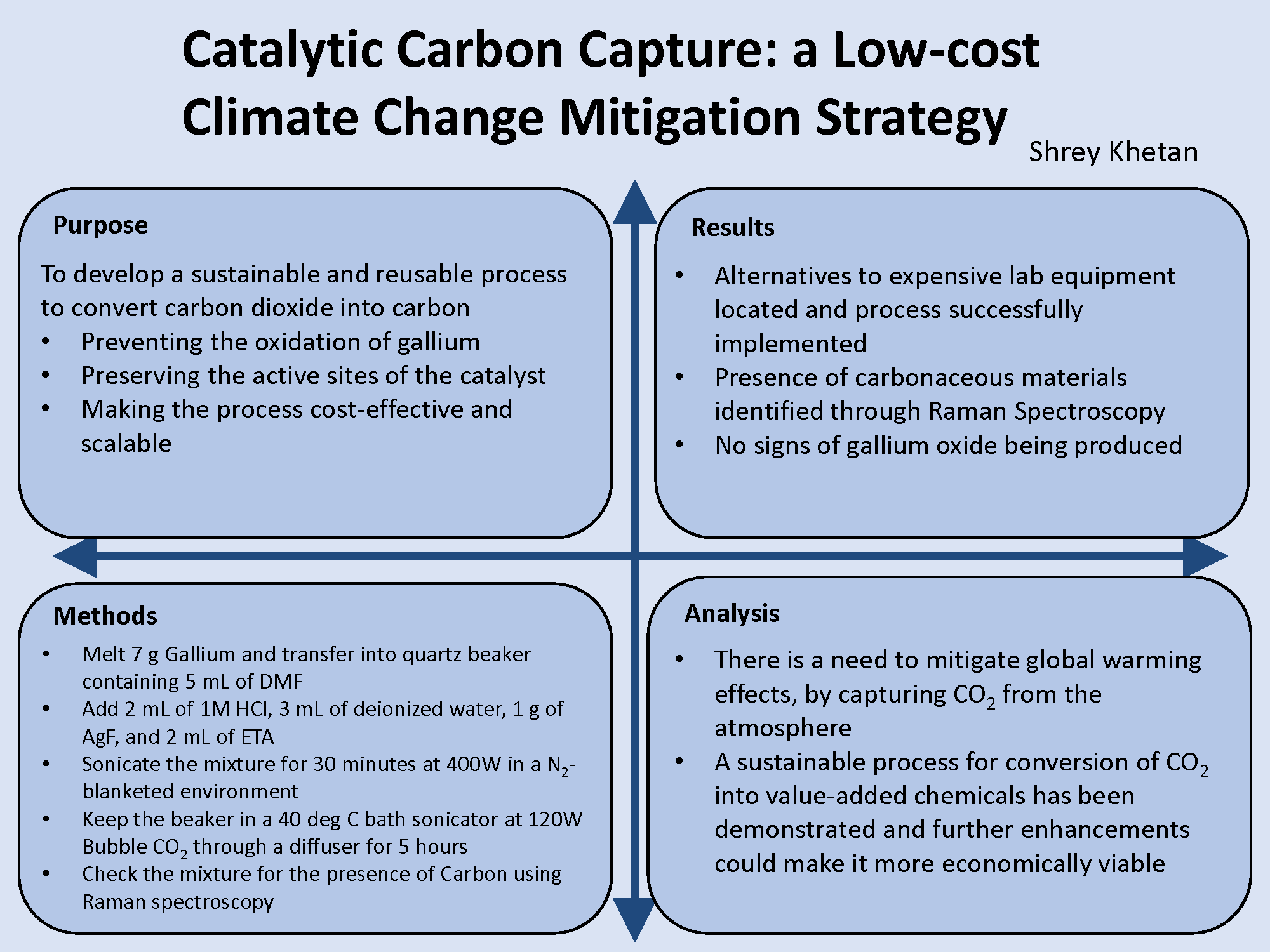
Climate change is driven by ever-increasing amounts of greenhouse gases like CO2. While newer technologies such as solar panels and electric vehicles are helping reduce the rate of increase of additional CO2 into the atmosphere, it is projected that these measures will not be enough to avoid major damage to global ecosystems. I am conducting research on the feasibility of converting carbon dioxide in the atmosphere to carbon as a means to help reverse the impact of global warming in the future.
After conducting a literature search for an economically viable method to capture carbon dioxide and convert it to carbon, I was able to find a research paper published in 2022 that appears to be simple in approach. The paper claims to have a sustainable way to keep bubbling carbon dioxide through a solution including a liquid metal catalyst and convert it efficiently into carbon. I have identified all the materials and methods needed to conduct the experiment. I also needed to think of alternatives to other items that weren’t readily available. Finally, I have built an experimental setup where the experiment would be safely conducted. I propose to explore the impact of varying process parameters like temperature to understand the impact on conversion. Further, I want to explore the mechanism of conversion of carbon dioxide to carbon. I propose to do this by bubbling carbon monoxide and see if it gets converted to carbon also, implying that CO could be an intermediate in the conversion process.
Procedure:
- Measure out 10 ml of DMF.
- Add 14g of liquid Gallium.
- Add 10ml of HCl.
- Add 2g of Ag(I)F.
- Add 4ml of ETA.
- Sonicate experiment using a probe sonicator for about 15-20 minutes with 15 seconds on and 3 seconds off.
- Move the experiment onto a hotplate magnetic stirrer and bubble CO2 while maintaining a temperature above 30 degrees Celsius.
- Conduct the experiment again with CO and varying parameters like temperature.
- Analyze carbon produced using spectroscopy.
Risk and Safety:
There are a few hazardous chemicals involved in this project like Dimethylformamide (DMF), Ethanolamine (ETA), Carbon Monoxide, and Hydrochloric Acid. To insure my safety I will use appropriate PPE (Personal protective equipment) and also do the experiment in a chemical fume hood.
Questions and Answers
1. What was the major objective of your project and what was your plan to
achieve it?
The main aim of my project was to explore a sustainable method of converting carbon dioxide
using liquid metal gallium catalysis, preserving the active sites of the catalyst and preventing the
oxidation of gallium. In order to achieve this objective, I first had to familiarize myself with
relevant literature and gather all required materials for the experiment. Then, I had to devise a
safe research plan that included identifying alternatives to expensive lab equipment and items
that were not readily available. Finally, I constructed an experimental setup to conduct the
experiment safely and have gone through a number of iterations to come up with a process that
demonstrated the presence of carbon upon bubbling CO2. As next steps, I plan to further
investigate the mechanism of this conversion and identify ways to optimize and scale-up the
setup.
a. Was that goal the result of any specific situation, experience, or problem
you encountered?
I am deeply concerned about the continuing and intensifying problem of global warming. I have
been researching for potential solutions for the mitigation of global warming that I could work
on for a research based class that I have been accepted to in my high school. I realized that
besides reducing the addition of CO2 into the atmosphere, researchers are working on Carbon
Capture and Utilization technologies where the existing CO2 in the atmosphere can be removed
or recycled. I had previously explored CO2 conversion to carbon using a Gallium-Indium
eutectic. This reaction was not sustainable and encountered the problem of gallium oxide being
produced over time, destroying the ability to catalyze the reaction.
b. Were you trying to solve a problem, answer a question, or test a
hypothesis?
I had previously demonstrated the possibility of converting CO2 to carbon using a
Gallium-Indium eutectic. However, I subsequently realized that the setup cannot sustainably
convert CO2 due to the problem of the oxidation of the gallium making the process not very
economically viable and sustainable. I set out to solve that problem using Gallium and Silver
fluoride Nanoparticle system that sustainably regenerate Gallium. I was trying to answer the
question “Is it possible to create a reusable catalytic system to convert carbon dioxide to solid
carbon?”. I continue to work to understand the reaction mechanistically by addressing the
hypothesis “Is Carbon monoxide an intermediate in the conversion of CO2 to carbon?”
2. What were the major tasks you had to perform in order to complete your
project?
● I read the relevant published literature and their supplements to identify all the materials
and methods needed to conduct the experiment.
● One of the major tasks I had to do was to figure out how I could get the probe sonicator
and the bath sonicator which can be very expensive instruments typically available only
in University labs. I tried a handheld probe sonicator of low power input before
eventually finding a reasonably priced sonicator with the right power input and with a
closed stand that was capable of sonicating materials for long periods of time.
● I also had to identify a local store providing a nitrogen tank for maintaining inert overlay
with an appropriately sized regulator.
● I was able to procure Gallium metal and other chemicals like Silver Fluoride, DMF and
ETA by ordering through my school’s chemical procurement.
● For carbon dioxide, I procured a CO2 cylinder and regulator through an Aquarium
supplies shop and was able to find a local shop that filled the cylinder with CO2.
● I had to identify a lab with a Raman Spectrometer microscope system that could help
analyze and verify carbon formation in carbonaceous samples generated in the reaction.
● Finally, I had to build an experimental setup where the experiment was safely conducted
for 5 hours.
a. For teams, describe what each member worked on.
Not applicable.
3. What is new or novel about your project?
I have attempted to develop a sustainable cost-effective process to convert carbon dioxide to
carbon using nano-particles of gallium and silver fluoride.
a. Is there some aspect of your project's objective, or how you achieved it
that you haven't done before?
The experimental setup of the Gallium and silver fluoride nanoparticle based CO2 conversion
system was a lot more complex than my initial effort last year to use a bubble column reactor
with Gallium-Indium eutectic alloy. I had to safely use chemicals like DMF and systems like the
Ultrasonic homogenizer and Nitrogen compressed gas. I also had to identify a lab that could help
verify production of carbon using Raman spectroscopy.
b. Is your project's objective, or the way you implemented it, different from
anything you have seen?
I was able to procure a probe sonicator and bath sonicator using the internet for a fraction of the
cost for high end models. I also had to get a N2 gas cylinder to create an inert environment while
sonicating. The procurement of chemicals like Dimethyl formamide were possible through the
assistance of my high school research class teacher.
c. If you believe your work to be unique in some way, what research have
you done to confirm that it is?
I performed a literary review on ways to convert CO2 to carbon. My project builds upon a
2022 paper and demonstrates a sustainable and high efficiency way to convert CO2 to
solid carbon. It is a simple approach that could be scaled up as a solution for effective
mitigation of greenhouse gasses.
4. What was the most challenging part of completing your project?
a. What problems did you encounter, and how did you overcome them?
I did not have the chemicals or the equipment setup to explore the sustainable conversion of CO2
to carbon. I had to procure the probe sonicator, the bath sonicator and the N2 gas cylinder. I also
had to identify a lab that had a Raman spectrometer that could identify if carbon was produced.
Initially I used a handheld sonicator of lower power to develop the nanoparticle dispersion and
used a magnetic stirrer bath for keeping the particles from coalescing while bubbling CO2.
However, this did not result in carbon formation. I subsequently got a higher power probe
sonicator with a insulted contained box and also a bath sonicator to keep gallium and silver
fluoride in suspension while bubbling CO2. When this enhanced setup was also not successful, I
contacted the authors of a 2022 paper and was told that having a N2 inert atmosphere is critical
while using a sonicator. It was only after I implemented this final step that I successfully detected
carbon.
b. What did you learn from overcoming these problems?
I learned that doing experiments that appear initially to be simple, actually require a lot of
preparation where equipment has to be bought and setup, and materials have to be procured
for experiments. I also learned that one may not get the conditions right in one go and iterative
experimentation with the right conditions are needed to make a reaction work. Finally, I also
learned that reaching out to people in their respective areas of expertise can be very helpful, like
conducting Raman Spectrophotometer analysis.
5. If you were going to do this project again, are there any things you would do
differently the next time?
I would try contacting authors of multiple papers and find out what they thought was absolutely
necessary for their experiments to run smoothly so I could avoid using equipment that won’t be
powerful enough like the handheld probe sonicator. This would have allowed me to directly go
after the more powerful box probe sonicator and use the bath sonicator with CO2 bubbled
through a diffuser instead of going through the initial magnetic stirrer system with open pipe CO2
sparging.
6. Did working on this project give you any ideas for other projects?
While CO2 reduction to carbon has been the focus of my efforts, similar reduction
systems can be applied to other greenhouse gases such as methane. Specifically for
CO2 reduction, I am interested in trying out the effect of other chemicals like magnesium
and cesium to see if it is more effective and higher efficiency rate.
7. How did COVID-19 affect the completion of your project?
Not applicable.