Role of S309-CAR-NK in Neutralizing SARS-CoV2
Abstract:
Bibliography/Citations:
- Borsa, M., Mazet, J.M. Attacking the defence: SARS-CoV-2 can infect immune cells. Nat Rev Immunol 20, 592 (2020). https://doi.org/10.1038/
s41577-020-00439-1 - Ghasemzadeh, Mehran, et al. “Exhausted NK Cells and Cytokine Storms in COVID-19: Whether NK Cell Therapy Could Be a Therapeutic Choice.” Human Immunology, U.S. National Library of Medicine,Jan. 2022, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8423992/.
- Gu, W., Gan, H., Ma, Y. et al. The molecular mechanism of SARS-CoV-2 evading host antiviral innate immunity. Virol J 19, 49 (2022). https://doi.org/10.1186/s12985-022-01783-5
- Ebrahimiyan H, Tamimi A, Shokoohian B, Minaei N, Memarnejadian A, Hossein-Khannazer N, Hassan M, Vosough M. Novel insights in CAR-NK cells beyond CAR-T cell technology; promising advantages. Int Immunopharmacol. 2022 May; 106:108587. doi: 10.1016/j.intimp.2022.108587. Epub 2022 Feb 9.PMID: 35149294.
- Barnes, Christopher O., et al. “SARS-COV-2 Neutralizing Antibody Structures Inform Therapeutic Strategies.” Nature News, Nature Publishing Group, 12 Oct. 2020, https://www.nature.com/articles/s41586-020-2852-1.
- Ma, Minh Tuyet, et al. “Car-NK Cells Effectively Target SARS-COV-2-Spike-Expressing Cell Lines in Vitro.” Frontiers, Frontiers, 30 June 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.652223/full.
- Pinto, D., Park, YJ., Beltramello, M. et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295 (2020). https://doi.org/10.1038/s41586-020-2349-y
- Furuse, Yuki. “Properties of the Omicron Variant of SARS-COV-2 Affect Public Health Measure Effectiveness in the COVID-19 Epidemic.” International Journal of Environmental Research and Public Health, U.S. National Library of Medicine, 19 Apr. 2022, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9099739/#:~:text=Although%20the%20short%20geneation%20time,the%20effectiveness%20of%20early%20isolation.
- Li, Rui, et al. “Evaluating the Impact of SARS-COV-2 Variants on the COVID-19 Epidemic and Social Restoration in the United States: A Mathematical Modelling Study.” Frontiers in Public Health, U.S. National Library of Medicine, 10 Jan. 2022, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8786080/.
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Innate Immunity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26846/
- “What Is Innate Immunity?” WHAT IS INNATE IMMUNITY? | Center for Innate Immunity and Immune Disease, https://ciiid.washington.edu/content/what-innate-immunity.
- “Structural and Functional Mechanism of SARS-COV-2 Cell Entry.” Abcam, 19 May 2022, https://www.abcam.com/content/structural-and-functional-mechanism-of-sars-cov-2-cellentry#:~:text=SARS%2DCoV%2D2%20S%20protein,to%20the%20host%20cell%20membrane.
- Di Vito, Clara, et al. “Natural Killer Cells in SARS-COV-2 Infection: Pathophysiology and Therapeutic Implications.” Frontiers in Immunology, U.S. National Library of Medicine, 30 June 2022, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9279859/.
- A., Angel, et al. “Graft versus Host Disease - Statpearls - NCBI Bookshelf.” Graft Versus Host Disease,10 Oct. 2022, https://www.ncbi.nlm.nih.gov/books/NBK538235/.
- “Cytokine Release Syndrome: Symptoms, What It Is & Treatment.” Cleveland Clinic, https://my.clevelandclinic.org/health/diseases/22700-cytokine-release-syndrome.
- Dietrich, Jorg, and Matthew j Frigault. “Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS).” UpToDate, 7 Dec. 2022, https://www.uptodate.com/contents/immune-effector-cell-associated-neurotoxicity-syndrome-icans#:~:text=Immune%20effector%20cell%2Dassociated%20neurotoxicity%20syndrome%20(ICANS%20is%20a,and%20T%20cell%20engaging%20therapies.
- Rivera, Drs. “Car T-Cell-Associated Neurotoxicity: Current Management and ... : Critical Care Nursing Quarterly.” LWW, Apr. 2020, https://journals.lww.com/ccnq/Abstract/2020/04000/CAR_T_Cell_Associated_Neurotoxicity__Current.7.aspx.
Additional Project Information
Research Plan:
Role of S309-CAR-NK in Neutralizing SARS-CoV2
Rationale:
In SARS-CoV-2, the variant’s properties influence the effectiveness of public health interventions, some even increase their transmissibility. Other properties of the variants are that they can also change the virus’s antigenicity (the ability of an antigen to induce an immunological response when it encounters the human body), avoid the immunity caused by previous infection/vaccination, and evade the immune response. All these characteristics increase the virus’s severity. For example, a variant of concern B.1.1.7 first identified in the UK was dominant in London and southeast England. It had the characteristic of being 50% more transmissible than previous variants. Omicron, characterized by 17 mutational changes, increased the number of cases dramatically by having a short generation time for transmission with a 59% higher transmissibility and 45% higher mortality rate, with a substantial antigenic change. The Omicron variant has efficient growth in the upper airway, and a bigger pool of children susceptible compared to adults, resulting in enhanced SARS-CoV2 evolution and declined vaccine efficacy. In the virus, there are two functional regions: S1 which consists of the receptor binding domain (RBD), and S2. S1 is responsible for the ability of the virus to bind to human cells to facilitate infection. Antigens against RBD have been seen as being able to stop the virus from binding to infect human cells. The spike proteins bind to the ACE2 receptors at the surface of healthy cells, then S2 is to fuse with the cell membrane of the healthy cell. During binding, S1 undergoes conformational rearrangement between up and down conformation states. The down conformation depicts how it hides the receptor binding domain (closed), and up exposes it but temporarily destabilizes the protein subunit (open). Antibodies for the coronavirus recognize and bind the spike protein to prevent the virus from infecting a healthy host cell. Neutralizing antibodies such as S309, isolated from a SARS patient, could diffuse not only this specific viral strain but also offshoots that occur because of natural mutations in the virus. S309 targets the RBD epitome which is mainly VH (Immunoglobin heavy chain variable region) mediated.
NK cells, also known as Natural Killer Cells, are a type of immune white blood cell that has granules that can destroy tumors and other virus-infected cells. Mature NK cells constitute about 10-15 % of circulating lymphocytes and are found in the spleen, bone marrow, liver (pit cells), lung, intestinal mucosa, uterus, and small amounts of lymph nodes. They can travel in and out of tissue and bloodstream and play a key role in the host’s immune defense against pathogens, preventing the establishment of infection and the viral spreading through the body. Their functions include releasing cytotoxic granules which punch holes in the target cell’s membrane by binding directly to phospholipids, creating pores, then releasing some molecules that get inside the target cell causing them to undergo apoptosis (type of program cell death). NK cells are affected in the bloodstream by SARS-CoV2, which SARS-CoV2 interrupts equations of immune responses, disrupting cytolytic antiviral effects of NK cells, and inducing a “cytokine storm” by activating infected immune cells. Adoptive transfer of proper cytolytic potentiated and lowest capacity of cytokine released NK cells and CAR-NK cells may be effective for this problem.
In a process like blood donation where a patient’s T cells are collected to reprogram T cells to produce special receptors on their surface known as chimeric antigen receptors (CAR) to recognize specific antigens on the cancer cells and destroy them. After the CAR-T cells are grown in a lab, they are infused back into patients. CAR-NK cells are a safer alternative to CAR-T cell therapy. Their advantages include less antigen loss relapse, minimal on-target, off-tumor toxicity, and antibody-dependent cellular cytotoxicity (ADCC). By having various activating and inhibitory receptors, it allows the CAR-NK cells to have stronger and less off-target reactions. Not only that, CAR-NK cells have a low risk of grafts versus host disease (GvHD), cytokine release syndrome (CRS), and immune effector cell-associated neurotoxicity syndrome (ICANS), where these side effects are often seen in CAR-T therapy due to allogeneic donors. This makes CAR-NK cells good candidates for “off-the-shelf” cellular immunotherapy treatment development because no evidence of GvHD is found in almost all clinical studies of NK cells. They release IFN-γ and GM-CSF that causes fewer toxic complications and reduces the occurrence of cytokine storms, making it a safe way of cell-based immunotherapy.
Given this information about SARS-CoV-2, their variants’ impact, especially Omicron, on public health, and the physical structure of the current variant of the SARS-CoV-2 virus, I’ve learned about the critical position the public is in. With this information along with learning about Natural Killer cells’ role in the immune system and S309-CAR-NK’s part in immunotherapy for this virus, I hypothesize that S309-CAR-NK cells can bind to Omicron subvariant XBB.1.5 Spike protein; therefore, neutralizing the pseudoviral SARS-CoV2 XBB.1.5 particles in vitro and can be used as a potential therapeutic for immunocompromised COVID patients. I will design these experiments as followed to test my hypothesis:
Research Questions:
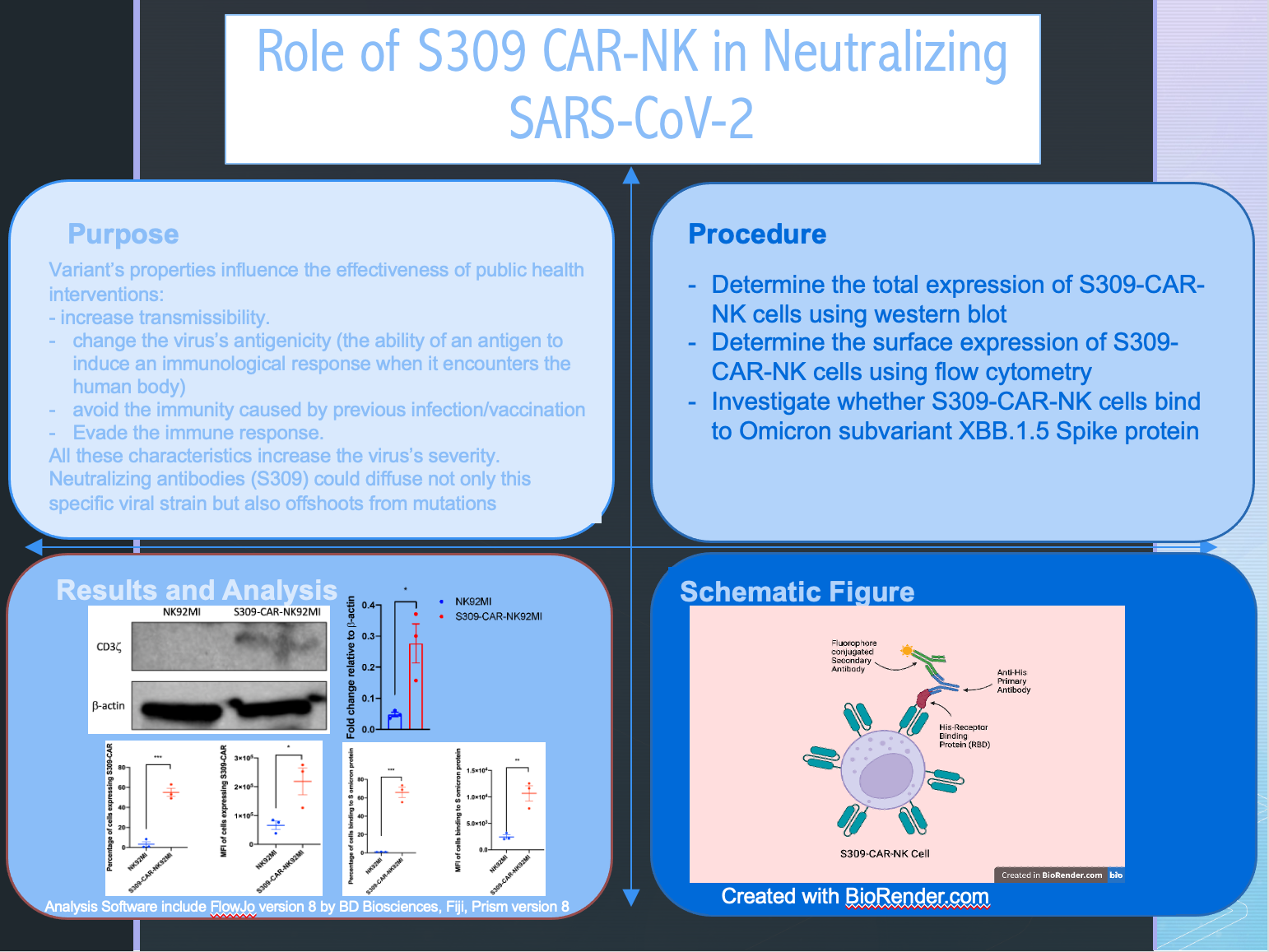
Do S309-CAR-NK cells express the S309 receptor?
Do S309-CAR-NK cells recognize the spike protein of Omicron variant XBB.1.5?
Do S309-CAR-NK cells bind to the spike protein of Omicron variant XBB.1.5?
Procedure:
- Determine the total expression of S309-CAR-NK cells using western blot:
- Add SDS and beta-mercaptoethanol into the protein and put protein into incubator to boil for 10 minutes
- Quantify protein concentrations using BCA kit.
- Load 20 μg of protein per lane. Using the micropipette (15 microliters) carefully add the protein sample and loading dye into the wells, using a buffer in between the chains.
- Fill the apparatus with running buffer.
- Attach the box's lid with the power supply and turn on the desired voltage. Watch to see if bubbles appear to be rising. Run gel until blue line reaches the bottom.
- Proteins now negatively charged because they are coated by the SDS. They will move from negative (top) to the positive terminal (bottom).
- Proteins stacked on top of the resolving gel and they resolve according to the function of their molecular weight
WESTERN BLOT
- While waiting for the gel, soak the sponges needed for western blotting in the transfer buffer.
- Cut pieces of the PVDF membrane and place them in a box to be treated with methanol and transfer buffer.
- Remove the gel once it’s done.
- Place sponge on the clear side of the chamber, then on top, add a piece of filter paper, then the gel, membrane, a piece of filter paper, and the sponge.
- Close the chamber with the “sandwich” inside.
- Place the chamber into a box and cover with transfer buffer. Add transfer buffer to the outer box, close the lid, and turn on the voltage at 100V for 1 hour in the cold room. Once it’s done, disassemble the module and place the membrane in a box.
- Pour the 5% milk blocking buffer over the membrane and place it on the rocker for 1 hour at room temperature.
- Add anti-CD3z primary antibodies at 1:1000 dilution and incubate at room temperature for 1 hour.
- Wash blot 5 times, 5 minutes each with PBST buffer.
- Add 1:2000 dilution anti-rabbit secondary antibody then incubate for 1 hour at room temperature.
- Wash blot 5 times, 5 minutes each with PBST buffer.
- Add ECL then expose the membrane using a Chemidoc
- Strip membrane for 15 minutes at room temperature then wash blot 2 times, 5 minutes each with PBST buffer.
- Add conjugated anti-β-actin (housekeeping protein) at 1:10,000 dilution and incubate at room temperature for 1 hour.
- Wash blot 5 times, 5 minutes each with PBST buffer.
- Add ECL then expose the membrane using a Chemidoc
- Determine the surface expression of S309-CAR-NK cells using flow cytometry:
- Parental non-modified NK cells will be used as negative control
- All centrifuging in protocol should be completed at 1250-1500 RPM or 300-500 g for five minutes.
- Label FACS tubes to be arranged in tube rack.
- Harvest cells and on last wash step resuspend the cells at 1 million cells/100 microliters of flow cytometry staining buffer (1x10^6/100μL)
- Aliquot 100 microliters into each facx tube
- Add conjugated antibody concentrated at 5-10 microliters per million cells ( 5-10μL/10^6) and vortex.
- CD56-PE-Cy7 for NK cells
- CD3-PE for T cells
- hIgG-APC for CAR marker
- Incubate cells for 30 minuets in the dark on ice.
- Wash cells in 2 milliliters of flow cytometry staining buffer to remove unbound antibody.
- Centrifuge the cells
- Vortex to resuspend the cells.
- Add 2 milliliters of flow cytometry staining buffer and repeat centrifugation.
- Resuspend cells
- In 200-400 microliters of flow cytometry staining buffer for flow cytometry analysis.
- Investigate whether S309-CAR-NK cells bind to Omicron subvariant XBB.1.5 Spike protein
- Parental non-modified NK cells will be used as negative control
- All centrifuging in protocol should be completed at 1250-1500 RPM or 300-500 GS for five minutes.
- Label facs tubes to be arranged in tube rack.
- Harvest cells and on last wash step resuspend the cells at 1 million cells/100 microliters of flow cytometry staining buffer (1x10^6/100μL)
- Aliquot 100 microliters into each facx tube
- Add 1 μg of Spike omicron protein. Vortex. Incubate on ice for 30 minutes.
- Wash cells with facs buffer.
- Add conjugated antibody concentrated at 5-10 microliters per million cells ( 5-10μL/10^6) and vortex.
- CD56-PE-Cy7 for NK cells
- CD3-PE for T cells
- hIgG-APC for CAR markers
- anti-RBD to detect spike protein binding to CAR-NK cells
- Incubate cells for 30 minuets in the dark at room temperature.
- Wash cells in 2 milliliters of flow cytometry staining buffer to remove unbound antibody.
- Centrifuge the cells
- Vortex to resuspend the cells.
- Add 2 milliliters of flow cytometry staining buffer and repeat centrifugation.
- Resuspend cells
- In 200-400 microliters of flow cytometry staining buffer for flow cytometry analysis.
Risk and Safety:
Biohazard chemicals include the use of sodium dodecyl sulfate (SDS), methanol, and beta-mercaptoethanol. To mitigate the risks of exposure, these chemicals will be handled inside a fume hood. As NK92MI is a cancer cell line and is derived from a cancer patient, additional precautions will be needed such as staining cells for flow cytometry in the hood. Personal protective equipment (PPE) including goggles, gloves, a lab coat, and closed-toe shoes will be always worn during experiments. The use of some equipment, such as a flow cytometer and heat block (used to denature proteins for the SDS-PAGE) may be dangerous and will be done by a supervised adult. All activities performed in the lab will be closely monitored by a supervisor adult.
Questions and Answers
1. What was the major objective of your project and what was your plan to achieve it?
Because the covid virus has been a big part of everyone's lives in recent years, the objective of my project was to reestablish how the S309-CAR-NK cells play a role in neutralizing the SARS-CoV-2 virus. The SARS-CoV-2 virus is a more recent virus and at the beginning, many developed vaccines failed to cure it. S309-CAR-NK cells were later introduced for this problem. By doing multiple experiments such as western blot, SDS-Page, and flow cytometry, I was able to find the intracellular expression of S309-CAr-NK cells, their surface expression, and whether or not they bind to the S omicron protein.
a. Was that goal the result of any specific situation, experience, or problem you encountered?
The goal was to experiment on a virus that has been a huge impact on everyone's lives and to test the hypothesis of the S309-CAR-NK cell's ability and role in neutralizing the problem many people have encountered.
b. Were you trying to solve a problem, answer a question, or test a hypothesis?
Test a hypothesis.
2. What were the major tasks you had to perform in order to complete your project?
The tasks I performed included reading a lot of research papers on this topic to familiarize myself with it, then using the western blot and SDS page to find the intracellular expression of the S309-CAR-NK cell. Then we proceeded to use flow cytometry to determine the surface expression of S309-CAR-NK cells and whether or not they bind to the virus (SARS-CoV-2 Omicron proteins). After the experiments, results were analyzed and conclusions were formed about the hypothesis that was tested.
a. For teams, describe what each member worked on.
Done individually
3. What is new or novel about your project?
Almost everything new about my project is to me. I had little lab experience and little knowledge about the project. But being passionate about it, I was exposed to a lot of research papers on this topic that helped me understand. The lab experiments were a little new to me as well, especially the use of flow cytometry. I've learned basic usages of lab equipment, but never performed such extensive experiments before. It was an amazing experience. Something else that is novel about my project is that usually CAR-T and CAR-NK cells are not what people usually talk about when it comes to infectious diseases. In my project, however, NK cells were definitely the center of the solution to this infectious disease.
a. Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
I had little access to the ability to perform experiments with cells and antibodies. I was able to achieve these experiments that were completely new to me with the help of Dr. Liu's lab student at Rutgers Cancer Research Lab. These experiments with western blot, SDS page, and flow cytometry were definitely new to me I have never had the chance to experience them.
b. Is your project's objective, or the way you implemented it, different from anything you have seen?
Yes, because I realize that CAR-NK cells are usually thought of to be used in immunotherapy and not with infectious diseases, so implementing it to the experiment for an infectious disease like the omicron protein was not something I had seen before.
c. If you believe your work to be unique in some way, what research have you done to confirm that it is?
At ClinialTrials.gov there is a group that uses "Nk cells Treatment for COVID-19 (NCT04280224)." In my experiment, I am using CAR-NK cells instead.
4. What was the most challenging part of completing your project?
I definitely had the hardest time going through all the information and research papers to familiarize myself with the project idea I was interested in. I also travel over an hour each time I needed and wanted to go to the lab for experimentation of analysis.
a. What problems did you encounter, and how did you overcome them?
Problems included the amount of time this project took up. With school and an extensive sports practice schedule, after homework, practice, and sometimes SAT, I dedicated a lot of time to the research project. I overcame the exhaustion by reminding myself of the passion I had for the topic to motivate myself.
b. What did you learn from overcoming these problems?
Being able to overcome these challenges allowed me to grow a lot as a student. It helped me with motivating myself, telling myself it is okay to be stressed, and it helped me a lot with my time management as well. As long as it is something that I want to do, I can always motivate myself to keep going even though the work was difficult.
HW and dedicated time
5. If you were going to do this project again, are there any things you would do differently the next time?
I think it would be a better idea for me to start my project a little earlier. I was a little slow at the beginning of the process, but towards the end, everything came out more organized. But I did have to work a lot because of the slow start.
6. Did working on this project give you any ideas for other projects?
Yes, this project was really interesting to me as I was able to learn more about something that was a huge part of everyone's lives. This infectious disease was something new that has developed recently and I would love to experiment on other possible infectious diseases or even sicknesses that have been a part of people's lives.
7. How did COVID-19 affect the completion of your project?
COVID-19 was the topic of my project, so it definitely had a great impact on my project. Restrictions-wise, I spent most of my time at home working on the papers and research or at the lab which I can only go to so often with caution and sanitation.