SALT & SURFACE: THE FASTEST WAY TO MELT ICE
Abstract:
Bibliography/Citations:
- https://ops.fhwa.dot.gov/weather/weather_events/snow_ice.htm#:~:text=Lanes%20and%20roads%20are%20obstructed,happen%20during%20snowfall%20or%20sleet, U.S. Department of Transportation
- https://www.uvm.edu/seagrant/outreach/road-salt-water-quality-salt-savvy-champlain#:~:text=How%20much%20salt%20do%20we,in%20the%20U.S.%20each%20year, Lake Champlain Sea Grant
- https://www.fhwa.dot.gov/pavement/sustainability/articles/pavement_thermal.cfm, U.S. Department of Transportation
- https://clearroads.org/project/20-02/ (First video: “Educational Video Describing the NaCl Phase Diagram) Clear Roads Research Program
- https://www.tf.uni-kiel.de/matwis/amat/iss/kap_6/illustr/i6_2_2.html, University of Kiel
- https://www.britannica.com/story/why-does-salt-melt-ice#:~:text=But%20you%20may%20be%20asking,will%20break%20into%20its%20elements, Encyclopedia Britannica
- http://hyperphysics.phy-astr.gsu.edu/hbase/Tables/thrcn.html, Georgia State University
- https://farside.ph.utexas.edu/teaching/316/lectures/node13.html#:~:text=Metals%20are%20good%20conductors%20(both,move%20freely%20throughout%20the%20metal, Farside Physics, University of Texas
- https://clearroads.org/project/20-02/ (First video: “Educational Video Describing the NaCl Phase Diagram) Clear Roads Research Program
Additional Project Information
Research Plan:
Materials
- filtered water
- 2 ice trays
- iodized salt
- measuring cup
- measuring spoons
- glass
- wood
- plastic
- metal
- stopwatch
- pen and paper
- phone camera
- freezer
Procedure
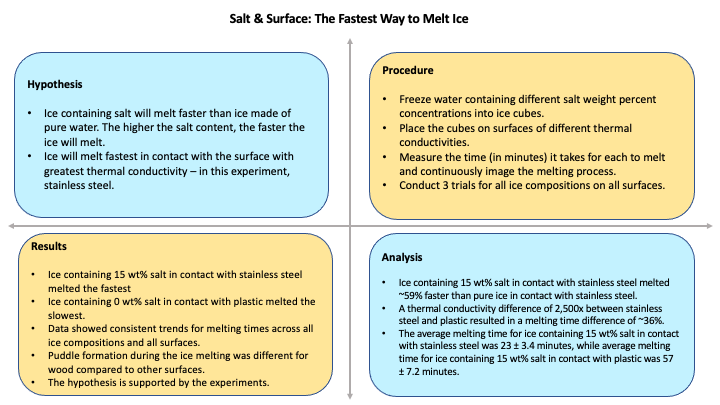
- Fill the measuring cup with 250 mL of filtered water.
- Pour 2 tsp of water into a slot in the ice tray.
- Repeat step 2 five more times for five other slots.
- Set the freezer’s temperature to 1°F. Carefully place the ice tray into the freezer for 4 hours.
- Remove the ice tray from the freezer. Remove four ice cubes and place one ice cube each on a different surface: glass, wood, plastic, and metal.
- Start the stopwatch.
- Take pictures every 5 minutes until the ice cubes finish melting.
- Note down the time it takes the ice cubes on each surface to completely melt.
- Stop the stopwatch after all the ice cubes are fully melted, which can be visually determined once all air bubbles are separated, and there is only a puddle of water visible.
- Empty out the ice tray and the measuring cup and dry the surfaces.
- Calculate 5 wt% salt for 250 mL of water:
- Create a ratio of the eutectic composition for salt water. 23.3 wt% of salt in water is equivalent to 2.54 lbs of salt in 1 gallon of water. 4
- Using this ratio, calculate the value of 5 wt% salt for 250 mL of water.
- Fill the measuring cup with 250 mL of filtered water.
- Add 5 wt% salt to water and mix vigorously until the salt is fully dissolved.
- Repeat steps 2-10 for the ice tray with the saltwater mix.
- Calculate 15 wt% salt for 250 mL of water using the procedure in step 11.
- Fill the measuring cup with 250 mL of filtered water.
- Add 15 wt% salt to the water and mix vigorously until the salt is fully dissolved.
- Repeat steps 2-10 for the ice tray with the saltwater mix.
- Repeat steps 1-18 two more times. Take the averages of all three trials and create graphs to show the trends.
Risk and Safety
This project does not require any safety procedures.
Questions and Answers
1. What was the major objective of your project and what was your plan to achieve it?
The major objective of my project was to understand the factors that affect the melting time of ice. To achieve this objective, I focused on two known factors: salt content in ice and the thermal conductivity of the surface ice is in contact with. By varying the salt contents and observing ice melting on surfaces with different thermal conductivities, I was able to complete the project.
a. Was that goal the result of any specific situation, experience, or problem you encountered?
In many parts of the U.S. and other countries around the world, de-icing of roads, sidewalks, and vehicles is a huge problem. I lived in Indiana for five years, and the streets were covered in so much ice that it was hard to walk in winter. It was so much of a safety issue that schools would be closed while waiting for the ice to melt. This motivated me to learn about the melting behavior of ice and how de-icing works.
b. Were you trying to solve a problem, answer a question, or test a hypothesis?
I was trying to answer questions related to the melting time of ice, including:
- How does salt content affect the freezing point of ice?
- How does the thermal conductivity of a surface affect the time ice takes to melt?
- What are some efficient ways to decrease the time ice takes to melt?
2. What were the major tasks you had to perform in order to complete your project?
There were four major tasks I had to perform to complete my experiment:
- I had to define the problem and create an experiment to try and understand it.
- I had to conduct the experiment in a methodical way and collect data.
- I had to analyze my findings and observe different trends.
- I had to study different concepts, such as the Freezing Point Depression and thermal conductivity to be able to understand and scientifically describe my results.
a. For teams, describe what each member worked on.
N/A
3. What is new or novel about your project?
I was able to study the effect of glass on the melting time of ice. Glass has a very similar thermal conductivity (0.8 W/mK) to concrete (typically 0.9-0.95 W/mK). Asphalt concrete is a commonly used material for road construction, so I was able to simulate the de-icing process of concrete, through using glass.
a. Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
This is the first time I have learned about concepts such as the Freezing Point Depression and thermal conductivity. I learned how to read phase diagrams and the effect of salt content in ice, and how that affects the melting time of ice. I also learned why different materials have different thermal conductivities through working on this project.
b. Is your project's objective, or the way you implemented it, different from anything you have seen?
In my project, I was able to combine both the effects of salt content in ice, and thermal conductivity of a surface that ice is in contact with, to study them concurrently. This is different from other projects I have seen which typically focus on one aspect or the other.
c. If you believe your work to be unique in some way, what research have you done to confirm that it is?
My work is in agreement with fundamental scientific concepts and what others have previously found.
4. What was the most challenging part of completing your project?
The most challenging part of completing my project was determining the exact time when an ice cube melted fully. I did not have equipment for this purpose, so I did it visually by making sure that the air bubbles were fully gone.
a. What problems did you encounter, and how did you overcome them?
At first, I used three different pieces of wood, but I had more varied results for melting times due to the non-uniformity of the surface. Then, I switched to using the same surfaces for each trial so the results would be consistent.
b. What did you learn from overcoming these problems?
I learned more about the effects of surface morphology on the time ice takes to melt, which sparked an interest for a future project.
5. If you were going to do this project again, are there any things you would you do differently the next time?
I would add on more materials, such as ceramics or granite. I would also change the salt weight percent further; for example, trying 1 wt% salt or 20 wt% salt. I would investigate how the melted water adjacent to the ice affects the subsequent melting time. Lastly, I would like to use better equipment for more precise determination of when ice has melted fully, such as an optical microscope.
6. Did working on this project give you any ideas for other projects?
Some ideas for new questions I got from working on this project include:
- Do other solutes, such as sugar, have the same effect on the melting point?
- If so, how does it compare to salt?
- What would happen if both salt and sugar were mixed in water? How would that affect the melting time?
- If there were windy conditions, how would air flow at the surface of ice affect the time take to melt?
Lastly, I would like to simulate what de-icing would really look like on concrete combining all of these ideas on a smaller scale. I would like to test other solutes with this to see what the most efficient way to melt ice really is.
7. How did COVID-19 affect the completion of your project?
Fortunately, COVID-19 did not have any effect on the completion of my project.