The Effect of Oxygen on the HOTHANDS Hand Warmer's Temperature
Abstract:
Bibliography/Citations:
- Greenberg, Julia. “The Chemical Reactions That Make Hand Warmers Heat Up.” Wired, Conde Nast, 26 Dec. 2014, https://www.wired.com/2014/12/whats-inside-hot-hands/.
- Poon, Twinkle. “The Chemical Magic of Hand Warmers.” Science Focus, http://sciencefocus.ust.hk/the-chemical-magic-of-hand-warmers.
- RALEIGH, G., et al. Air-Activated Chemical Warming Devices: Effects of Oxygen and Pressure. Center for Comprehensive Wound Care and Hyperbaric Medicine, St. Luke’s Medical Center, Milwaukee, WI 53215, https://www.uhms.org/images/Safety-Articles/air_activated_warmers_raleig.pdf.
- Science Buddies Staff. "Bring on the Heat! Investigating Exothermic Reaction Rates." Science Buddies, 20 Nov. 2020, https://www.sciencebuddies.org/science-fair-projects/project-ideas/Chem_p054/chemistry/investigating-exothermic-reaction-rates. Accessed 24 Oct. 2022.
- Wang, Linda. “What’s inside Disposable Hand Warmers? Small Packets of Warmth Work through a Simple Exothermic Reaction.” Cen.acs.org, Volume 88, Issue 4, Chemical & Engineering News, 25 Jan. 2010, https://cen.acs.org/articles/88/i4/Hand-Warmers.html.
Additional Project Information
Project files
Research Plan:
Background Research
HotHands® Hand warmer heat packs are available in most grocery and drug stores. Each hand warmer pack contains iron powder, water, polymer, Vermiculite, activated carbon (Charcoal), and salt. When the pouch is taken out of its outer packaging, oxygen in the air moves across the pores in the pouch cover. With salt and water available, the oxygen reacts with the iron powder inside to create iron oxide (Fe2O3) and release heat. (Wang). as shown in the chemical equation below:
4 Fe (s) + 3 O2 (g) → 2 Fe2O3 (s) + heat energy
Iron powder is used because the large surface area of the fine powder greatly speeds up the oxidation reaction. The sodium chloride acts as a catalyst to speed up the reaction rate. Charcoal is available to separate the heat built up and to draw in odors. Chloride ions in the salt act as catalysts to further accelerate oxidation. The salt acts as a catalyst, the carbon helps disperse the heat, the vermiculite is used as an insulator to retain the heat, and water is required for the reaction. All of these ingredients are surrounded by a polypropylene bag that lets air in but contains moisture.
The chemical reaction is oxidation and because it generates heat, it’s also an exothermic reaction.
The temperature attained by a warming device largely relies on the rate of the oxidative process. In chemistry, the law of mass action states that the rate of a reaction is proportional to the product of the concentrations of the participating molecules. Thus, if the concentration of oxygen is increased, the rate of the reaction will also increase. As the reaction rate increases, so does the amount of heat produced.
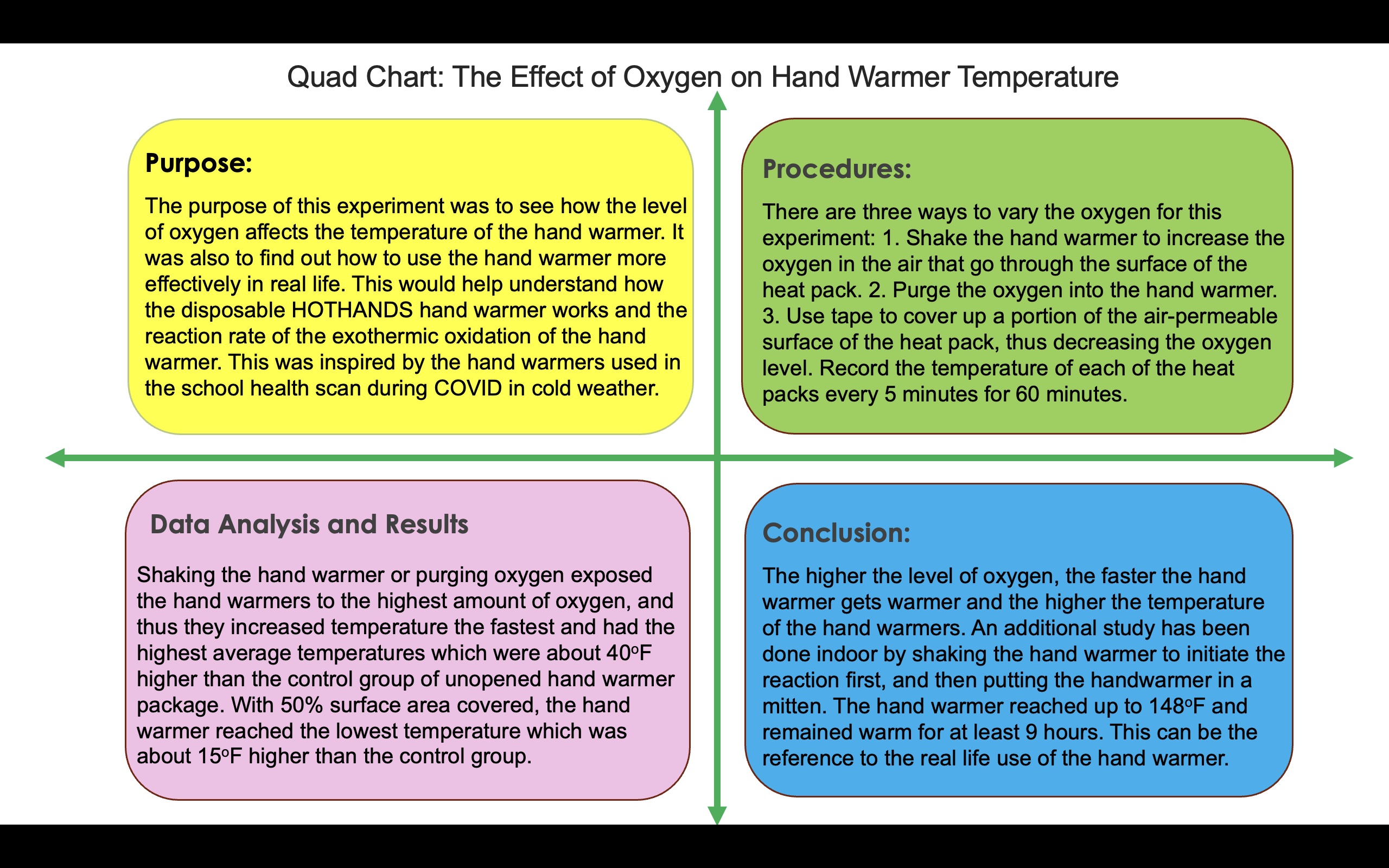
With this research in mind, we can design the experiment to demonstrate that increasing the amount of oxygen available to react with the iron will increase the amount of heat produced by the exothermic reaction. There are three ways to vary the oxygen for this experiment:
1. Shake the hand warmer to increase the oxygen in the air go through the surface of the heat pack and react with the iron
2. Use the oxygen in the oxygen canister to purge the oxygen into the heat pack
3. Use tape to cover up a portion of the air-permeable surface of the heat pack, thus decreasing the oxygen in the air getting through the heat pack.
Materials:
- HotHands® hand warmer packs (8)
- Infrared (IR) thermometer
- Oxygen canister with 99.7% Pure Oxygen, and spray (4L x2)
- Label of numbers 1,2,3,4,5,6
- Tape
- Timer
- Lab notebook
- Disposable glove
Procedures:
- Preparing the Heat Packs
Using the masking tape and permanent marker, label the heat packs 1 to 5:
#1: Unopened package
#2: 50% surface area open, air, no pure oxygen
#3: 100% surface area open, air, no pure oxygen
#4: 100% surface area open, shaken in the air, no pure oxygen
#5: 100% surface area open, pure oxygen level 1
#6: 100% surface area open, pure oxygen level 2 (double oxygen level 1)
- Follow the procedure below to record the time and temperatures of the heat packs.
- Place an unopened heat pack as #1. This is a control to track room temperature.
- Open the wrapper for heat pack #2-6 and place it on the table, with the air-permeable side facing up.
- Open the wrapper for heat pack #2 and cover 50% of the perforated surface with tape.
- Open the wrapper for heat pack #4 and ask a helper to start to shake it in the air.
- Gently spray the oxygen over the surface of heat packs #5 and #6. The frequency of spraying the oxygen over the surface of heat pack #6 is twice of that heat pack #5.
- Use the timer for all of the readings, from the time of start until the experiment is complete.
- Record the time the temperature was measured.
- Record the temperature of each of the heat packs every 5 minutes for 60 minutes.
- Record each temperature three times and calculate the average.
- Record all data in the lab notebook.
Questions and Answers
1. What was the major objective of your project and what was your plan to achieve it?
The major objective of my project was to investigate how the disposable HOTHANDS hand warmer works and to understand the reaction rate of the exothermic oxidation of the hand warmer. It was also to find out how to use the hand warmer more effectively in real life. I planned to achieve this by controlling the level of oxygen getting through the hand warmer surface and measuring the temperature of the hand warmers over a period of time to see how the level of oxygen affects the temperature of the hand warmer.
a. Was that goal the result of any specific situation, experience, or problem you encountered?
This was inspired by my family’s donation of hand warmers to the school during COVID. Last year when school reopened after winter break, it was extremely cold, but the school administrators still had to do the health screening scan outside of the building every morning. My family donated two boxes of hand warmers to the school and they helped the teachers feel warmer with their hands. I used a couple of them and found that they worked very well and I was very curious why a small pack worked like magic and could last a very long time. So I did some online search and research to find out the chemistry behind it and wanted to explore more to understand the exothermic reaction and the mechanism of reaction rate.
b. Were you trying to solve a problem, answer a question, or test a hypothesis?
In this experiment, my goal was to test the hypothesis of whether increasing the amount of oxygen going through the hand warmer surface would increase the temperature of the hand warmer as well as the rate of temperature rising which is caused by the heat generating of the exothermic reaction.
2. What were the major tasks you had to perform in order to complete your project?
To complete my project, I had to first do market research to find the most convenient and affordable infrared thermometer and oxygen canister because of my budget and safety concerns. After I collected all the tools to use, I had to practice using them to reduce human errors. Since I found the most user-friendly tools, I was able to conduct the experiment mostly by myself with minimum help from a partner.
a. For teams, describe what each member worked on.
3. What is new or novel about your project?
Despite other setup and hand warmer testing experiments that you can find online, I tried to simplify the experiment not only due to the budget limit but also because I believe in the beauty of being simple and straightforward. In chemistry experiments, the simple procedure also means minimizing the safety risks. So I found the oxygen canister which is very affordable and easy to use.
a. Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
b. Is your project's objective, or the way you implemented it, different from anything you have seen?
c. If you believe your work to be unique in some way, what research have you done to confirm that it is?
Overall, this project was my first time designing and completing a science project by myself. Compared to the other similar procedures I see, the major difference is how to get pure oxygen that I can easily purge into the hand-warmer surface. (see 4a below)
Another difference is that most procedures use containers to hold the hand warmers, and I simplified putting the hand warmers on the top of the tables. Because the main objective of the experiment was to prove the hypothesis of the effect of oxygen on the hand warmer temperature, it doesn’t matter if the hand warmers are in a container or not, as long as the table surface that holds the hand warmers is the constant in the experiment. Later I did an additional study to mimic the real-life usage of the hand warmer in the mitten as the supplementary experiment to answer my question about how to use the hand warmer most effectively in real life.
4. What was the most challenging part of completing your project?
The most challenging part of completing my project was being very busy during the experiment to do all the measuring and recording one after another every 5 minutes with limited help from others. With more help from partners, the experiment would be able to last a long time, then more data would be available, especially till the time the temperature started to drop.
a. What problems did you encounter, and how did you overcome them?
The major challenge was to find the oxygen canister. I visited several hardware stores looking for the traditional oxygen canister with regulator and hose suggested by a science project website, which can be purchased as part of a torch kit from most hardware stores. But the hardware stores only had the propane part of the torch, not the oxygen part. And the store staffs were all concerned with the purpose of the oxygen. So I did more online searching and found out that many convenient home-use oxygen canisters are available due to the pandemic. So I bought the type with the spray which is easy to handle and perfect for my project.
There was a random error with the temperature measuring. The size of the hand warmer is about 6cm x 8cm. The temperature of the hand warmer at each spot in this area is not consistent and may fluctuate unexpectedly. An increase in replicates can improve this. But due to the limited manpower, I could only measure each temperature 3 times and calculated the average. But I improved it by using a marker to mark the spot for temperature measure so for each hand warmer, the temperature was measured on the same spot every time.
b. What did you learn from overcoming these problems?
I learned that simplicity is key and that using the right tools can save lots of manpower and make the experiment easy to handle.
5. If you were going to do this project again, are there any things you would do differently the next time?
Next time, I would try to use a vibrator to shake hand warmer #4 because shaking the hand warmer #4 for an hour was very tiring if done all manually. The foot massage vibrator at my home was not good for this purpose because the shaking was not vigorous enough to make a difference. If I had more budget, I’d use a lab shaking plate. With the help of the shaking plate, I can extend my experiment to several hours till the hand-warmer temperatures start to decrease.
6. Did working on this project give you any ideas for other projects?
This experiment made me more interested in the other types of hand warmers, especially recycled hand warmers which sound more eco-friendly. They are different mechanisms so I’d like to investigate that and do some experiments to see how the reaction can be reverted.
7. How did COVID-19 affect the completion of your project?
Because I did the experiment indoors at home, COVID-19 did not affect my experiment. But maybe due to COVID-19, there are a lot of home-use oxygen canisters available for purchasing online, which was a major improvement of my experiment design and significantly simplified the experimental procedure.