Corrode Much?
Abstract:
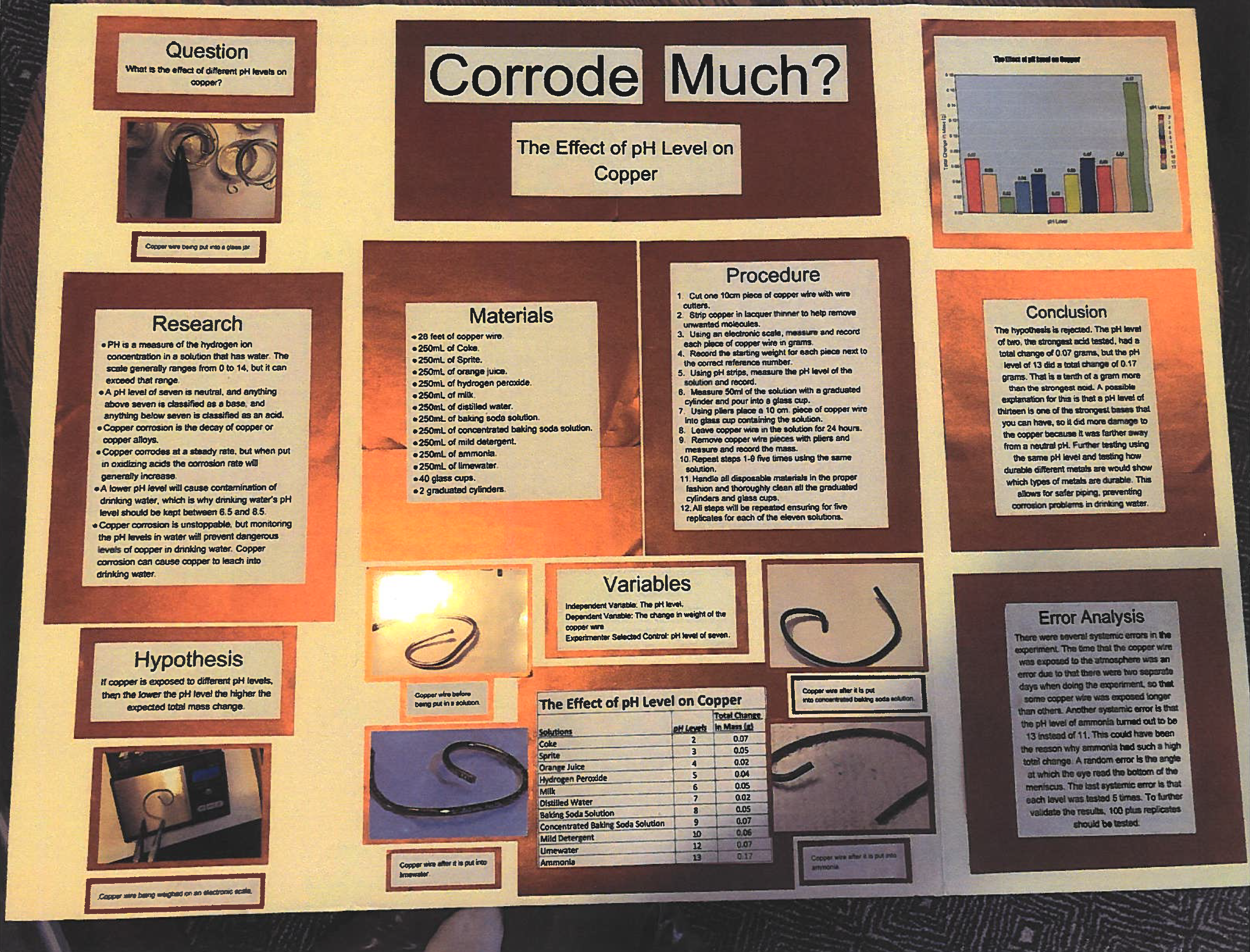
Do different pH levels affect copper wire differently
The purpose of this project was to determine if different pH levels have any effect on copper wire. A total of 55 copper wire pieces were used with their pre-weight recorded. There are 11 solutions, each with five replicates. Each copper wire piece was put into the same amount of the solution, had the same temperature and climate, were in their for the same amount of time, and were measured by the same electronic scale. The hypothesis was if copper is exposed to different pH levels, then the lower the pH level, the higher the total expected mass change. The independent variable in this experiment is the pH level, and the dependent variable is the total mass change. My experimenter selected control was the pH level of seven. The dependent variable was measured by an electronic scale and adding up the mass change for all five replicates. My results were that while that the lowest pH level was tied for second most change, my highest pH level actually had the most total change. My lowest pH had a change of 0.07 grams, and my highest had a change of 0.17. Based on these results, the hypothesis is rejected. If I did this experiment again, I would test different metal types on the same pH level instead of different pH levels on the same type of metal.
Bibliography/Citations:
No additional citationsAdditional Project Information
Research Plan:
Title: Corrode Much?
Question: What is the effect of different pH levels on copper wire?
Hypothesis: If copper is expose to different pH levels, then the lower the pH level, the higher expected total mass change.
Material: 28 feet of copper wire, 250 ml of Coke, Sprite, orange juice, hydrogen peroxide, milk, distilled water, baking soda solution, concentrated baking soda solution, mild detergent, ammonia, limewater, 40 glass cups, 2 graduated cylinders, and 100 pH level testing strips.
Procedure:
- Cut one 10 cm piece of copper wire with wire cutters.
- Strip copper in lacquer thinner to help remove unwanted molecules.
- Using an electronic scale, measure the piece of copper wire in grams.
- Record the starting weight for each piece next to the correct reference number.
- Using pH strips, measure and record the the pH level of the solution.
- Measure 50 ml of the solution with the graduated cylinder and pour into a glass cup.
- Using pliers place a 10 cm piece of copper wire into a glass cup containing the solution.
- Leave copper wire in the solution for 24 hours.
- Remove copper wire pieces with pliers and measure and record the mass.
- Repeat steps 1-9 five times using the same solution.
- Handle all disposable materials in the proper fashion and thoroughly clean all the graduated cylinders and glass cups.
- All steps will be repeated to ensure for five replicates for each of the eleven solutions.
Data Analysis: Record all copper wire pieces after 24 hours in their respective solutions. The total mass change will be calculated. A bar graph with all eleven pH levels will be on the x axis and total mass change will go on the y axis.
Risk Analysis: Always wear gloves and eye protection. Extra caution should be used with ammonia.
Works Cited:
Bradley , David. “Corrosion Isn’t All Bad.” Corrosion Isn't All Bad, Feb. 2006,
www.reactivereports.com/52/52_2.html.
“Corrosion of Copper and Copper Alloys.” Corrosion off Copper and Copper Alloys,
June 2001, www.totalmateria.com/Article16.htm.
Helmenstine, Anne Marie. “The PH Level in Chemistry Distinguishes Acids From
Alkalines.” ThoughtCo, ThoughtCo, 7 May 2019,
www.thoughtco.com/definition-of-ph-in-chemistry-604605.
“PH in Drinking Water.” PH in Drinking Water, May 2007,
www.watersystemscouncil.org/download/wellcare_information_sheets/potential_ground
water_contaminant_information_sheets/9709284pH_Update_September_2007.pdf .
Photos by Entrant and/or photos by entrant’s parent or guardian.
“What Is Copper Corrosion? - Definition from Corrosionpedia.” Corrosionpedia, www.corrosionpedia.com/definition/1642/copper-corrosion.