BioClean - A new innovative biological method for removal of NOx pollutants from the atmosphere.
Abstract:
AbstractThe goal of this project is to find a way to remove the NOx emission from air particularly that emitted from automobile exhaust. More than 4.2 million people die from air pollution every year. Most of these deaths come from ground level ozone and NO2. In the presence of sunlight, NO2 catalyzes the formation of ozone from oxygen. NO2 and ozone can cause asthma and other respiratory problems. Currently, the available methods for NO2 removal are catalytic converters for automobiles or massive scrubbing devices for industrial processes. Even though catalytic converters remove most NOx some still get into the environment from automobile exhaust and other sources. Can we capture the NOx being released into the air and convert them to natural, harmless gases? The purpose of this project is to develop a new innovative method for capturing the NO2 from the air and use biological methods for conversion to nitrogen.
The project consists of three parts, a method for capturing the NO2 from the atmosphere, converting it to nitrite and nitrate salts and a method for converting the salts to nitrogen gas using bacteria and algae. To store the NO2, it was adsorbed onto activated carbon. Using around 50mL of carbon in a prototype adsorption device, it took an hour and forty-five minutes for the carbon to be saturated. An arduino based sensing device was used to measure the NO2 emission. When the carbon was saturated, it was taken from the adsorption device and transferred to a flask. Steam was passed through the flask, liberating the NO2. The steam and NO2 were bubbled through cold water to condense the steam and dissolve the NO2. This creates a mixture of nitrous and nitric acid. When these acids were reacted with sodium hydroxide, it created a mixture of sodium nitrite and nitrate. The acid, and consequently the salt, are so dilute that most of the water had to be boiled off before the nitrite level reached 0.5 ppm. However, if done with more carbon on a larger scale, this could create more nitrite and nitrate salts. These salts can disrupt ecosystems so they need to be destroyed. Nitrifying bacteria like nitrobacter can convert nitrites to nitrates. Denitrifying organisms like cyanobacteria can then convert the nitrate to nitrogen and oxygen gas. For the denitrifying organisms, spirulina algae was used. To measure the algae growth, a simple arduino based photospectrometer was designed. The experiment was successful and although this method needs more tests, it could be used to remove NO2 from the atmosphere.
Bibliography/Citations:
No additional citationsAdditional Project Information
Research Plan:
Project Name
BioClean - A new innovative biological method for removal of NOx pollutants from the atmosphere.
Project Question
More than 4.2 million people die from air pollution every year. Most of these deaths come from NO2 and ground level ozone. In the presence of sunlight, NO2 catalyzes the formation of ozone from oxygen. NO2 and ozone can cause asthma and other respiratory problems. Currently, the only available methods for NO2 removal are catalytic converters in automobiles or massive scrubbing devices in industrial processes. Even though catalytic converters remove most NOx some still get into the environment from automobile exhaust and other sources. Can we capture the NOx being released into the air and convert them to natural, harmless gases? The purpose of this project is to develop a new innovative method for capturing the NO2 from the air and use biological methods for conversion to nitrogen.
Rationale
NOx is a dangerous air pollutant. NOx and ozone are responsible for 1 death every 10 seconds. This is a major global problem upon which action must be taken. To understand how to solve the air pollution problem, we must first understand what we need to remove. Table 1 shows all known NOx compounds (EPA, 1999).
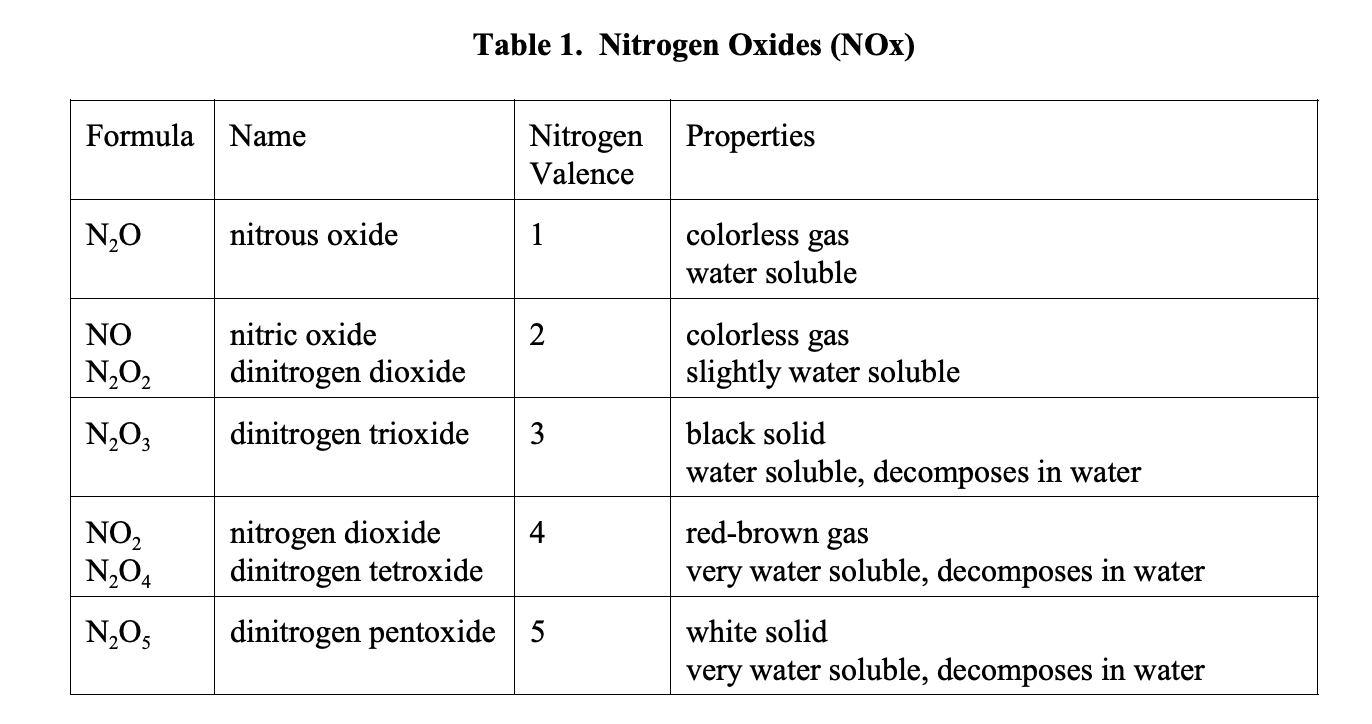
N2O is a biogenic compound and an ozone depleting chemical. It is naturally occurring, but it is largely generated by humans from farming. It reacts with O3 in the upper atmosphere to create NO. NO is quickly converted into NO2 when it reacts with O2. The reactions are as follows.
![]()
![]()
In the past, before the industrial revolution, atmospheric N2O levels were low and so were atmospheric NO2 levels. However, after the industrial revolution, N2O levels have skyrocketed.
NO is formed when molecules of N2 and O2 decompose at high temperatures and bond to form NO. Most anthropogenic NOx emissions are NO from high temperature combustion. When NO is released into the atmosphere, it readily combines with O2 to form NO2. These reactions can be written out as so.
![]()
![]()
NO2 is a dangerous chemical whose exposure can cause lung cancer and other respiratory problems. It is created from many sources, including welding and NO2 can create O3 if it is photocatalyzed, heated, or reacted with VOC (volatile organic compounds) radicals. Then, it will break down into NO and O. The O quickly reacts with O2 to form O3, which is highly toxic at ground level. Ozone is toxic by itself, but when it reacts with normal household products it releases toxic VOCs like formaldehyde and benzene. These chemicals are highly toxic and contribute to millions of premature deaths worldwide.
![]()
![]()
![]()
These equations can be simplified to one equation,
![]()
As you can see, the NO2 in this equation converts O2 to O3, but in the end NO2 is regenerated, ready to restart the cycle all over again with more oxygen molecules. Because of this, just curbing NO2 emissions will not solve the problem. NO2 has a long lifespan and will continue to create ozone from oxygen for its entire life. We need to take an initiative to remove NO2 from the atmosphere as well as curb our emissions.
Introduction
The goal of this project is to develop a new innovative method to remove NO2 from the atmosphere using a three step process -
- NO2 capture using adsorption
- Conversion of NO2 to nitrite and nitrate salts
- Conversion of nitrites and nitrates to nitrogen gas using a combination of bacteria and algae.
The NO2 will be captured from the atmosphere using activated carbon. Then it will be desorped using one of two methods, passing steam through it, or refluxing it. The experiment will determine which of these methods are better. This will create a mixture of nitric and nitrous acid. The temperature and other external conditions such as UV light concentration can all contribute to the type of acid and whether or not small amounts of nitric oxide is produced. After that, the acids will be reacted with sodium hydroxide. This will create nitrite and nitrate salts. These will be fed to a mixture of bacteria (nitrobacter) and algae (Spirulina). These will destroy the salts and convert them to nitrogen gas.
The ways that NO2 can absorb are shown below (Fang et al., 2019)
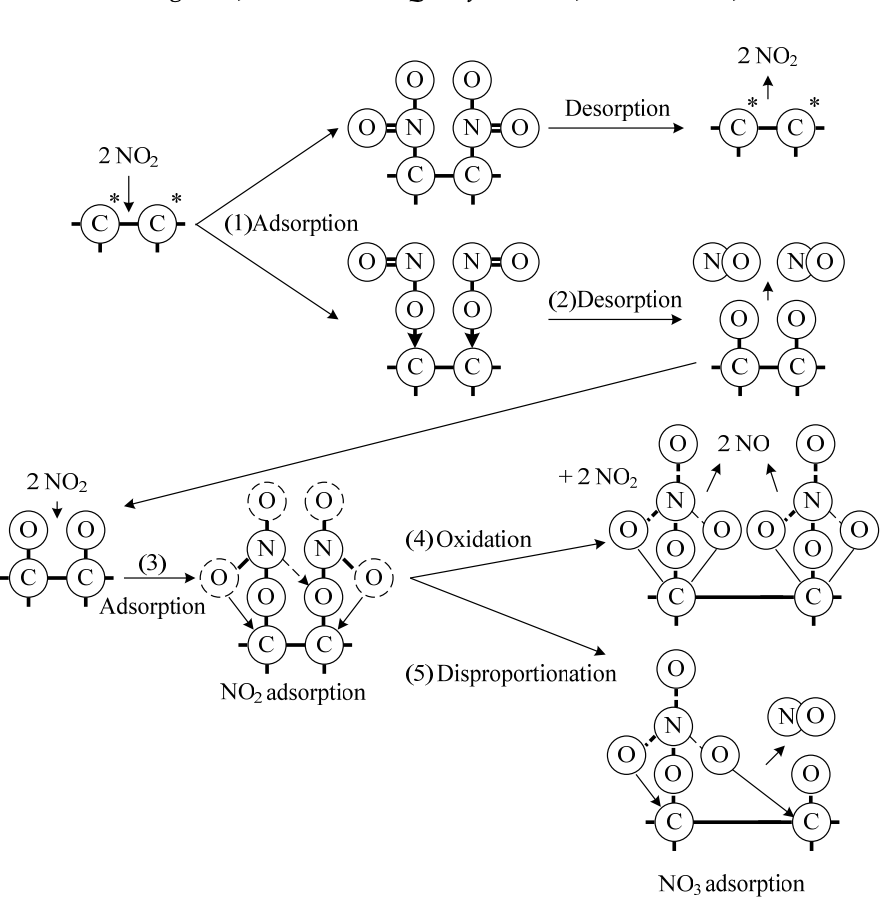
As the NO2 adsorbs, it can form many different bonds with the carbon, and in some cases oxidizing the carbon. While most of the NO2 is adsorbed, some of it can desorp spontaneously as NO2 and NO. This means that the process is not completely efficient, and is also why the carbon must be stored in a stoppered container to prevent the gas from escaping.
To desorp the NO2, most papers recommend the use of a flow reactor. I do not have access to a flow reactor. I will try to reflux the charcoal in water to see if I can add enough energy to break the carbon-NO2 bonds. In this case, the carbon will be submerged in water so any NO2 gas that is liberated will dissolve into the water. The other method is steam desorption. However, this method has never been tried with NO2, only with sulfur based products (Kamino et al., 1972).
The acidic solution will be treated with sodium hydroxide. This process creates nitrites as waste. Nitrobacter oxidizes the nitrite ion into the nitrate ion (Ferguson and Nicholls, 2013). To do this, it first reacts water with the ion. The oxygen is transferred to the nitrite ion, converting it to nitrate. This reaction leaves 2 hydrogen ions and two electrons. These ions can then be used by the cell's mitochondria to produce energy. The oxidation reaction is as follows,
![]()
The hydrogen plus ions and the two electrons can be reacted with oxygen gas from the atmosphere to produce water and energy. The reaction is as follows,
![]()
These reactions combined look like this,
![]()
This reaction gives ![]() of energy per mole of nitrite.
of energy per mole of nitrite.
As well as using nitrite as an energy source, nitrobacter is also a carbon sequestering bacteria, taking in carbon dioxide to fulfill its carbon needs. This makes it an optimal choice for nitrite removal.
To destroy the nitrate, cyanobacteria (Spirulina) will be used to convert it to N2 gas (Bothe et al., 2007). The equation can be written out as follows.
![]()
This reaction has four parts to it. First, the nitrate is converted to nitrite. This causes the combination of electrons, hydrogen cations, and the liberated oxygen to produce water. This charge difference allows the cell's mitochondria to synthesize ATP. Then, the nitrite is converted to nitric oxide. This liberates still more oxygen, creating water and allowing ATP synthesis. After that, two molecules of NO are converted to one molecule of N2O, nitrous oxide. This liberates yet another oxygen which the cell uses in the same fashion. The final step is taking the oxygen from the nitrous oxide, converting it to nitrogen gas and using the oxygen for energy. This process can release small amounts of NO and N2O, however, it is a very small amount and both NO and N2O can be reused by other algae to produce N2 and energy.
Engineering Goal
The purpose of this project is to develop a new innovative method for NO2 removal from the atmosphere using biological processes.
The process will have three phases
- NO2 adsorption using activated charcoal
- method to convert the NO2 to nitrite and nitrate salts
- conversion of the nitrite and nitrate to nitrogen gas using nitrobacter and algae.
Procedure
Part 1: Adsorption
- Cut a large piece of PVC pipe into 5 small segments and one large segment.
- To make part one of the adsorption device, connect the parts in the following order:
- Heat proof tubing.
- Small PVC segment.
- 3 way adapter.
- On one hole of the 3 way adapter, attach a small PVC segment, then attach the male screw adapter.
- On the other hole, attach a small PVC segment.
- 3 way adapter
- Small PVC segment.
- Female screw joint.
- To make part two, connect the following parts:
- Male screw joint.
- Large PVC pipe segment.
- Female screw joint.
- To make part three, connect the following parts:
- Male screw joint.
- Small PVC segment.
- 3 way adapter.
- Add heavy duty epoxy resin to all of the connections.
- Wait overnight for the epoxy to cure and set.
- The next morning, remove the rubber washer from the inside of female adapters. Place a piece of window mesh where the washer was, then put the washer back.This creates a mesh to hold the charcoal in place.
- Screw the valve to the male adapter connected to the 3 way adapter in part one. Be careful not to twist too hard or the epoxy may break.
- Then, fill part two with activated charcoal.
- Tightly pack the charcoal and fill again.
- Continue step 9 until no more charcoal can be packed into the tube.
- Screw part two into part one, not letting any charcoal fall out.
- Screw part three into part two.
- Put stoppers in the open three way adapters.
- Move the snowblower into an open area away from other people.
- Set up two ladders with a board in between them. Make sure the board is level with the snowblower's exhaust.
- Place the adsorption device on the board.
- Get an extension cord and connect the snowblower and arduino based NO2 sensor to it. The arduino based sensor was built as a side project to measure the NO2 concentration.
- Make sure the snowblower has a full tank of gas and oil, this experiment could run for over an hour.
- Connect the adsorption device to the snowblower by connecting the heat proof tubing to the snowblower exhaust pipe.
- Switch on the snowblower.
- Remove the stopper from the first 3 way adapter and place the sensor probe in the hole.
- Follow the on-screen prompts to take the untreated gas measurement.
- Replace the stopper in the front 3 way adapter and remove the stopper from the back 3 way adapter.
- Put the sensor probe in the 3 way adapter and follow the on-screen prompts to begin the test run.
- Wait until the NO2 concentration coming out of the device is equal to the concentration of NO2 in the original exhaust. This means that the carbon is fully saturated and is not adsorbing any more NO2.
- Wait for the setup to cool down, then unscrew part two from part one. Empty the resulting charcoal into a flask.
- Remove the ladders, board, extension cord, and snowblower.
- Remove the microSD card from the sensing device.
- Upload the data from the SD card onto a computer and store the charcoal in a stoppered flask.
Part 2: Desorption and conversion to nitrite and nitrate.
- Divide the charcoal up into 2 different samples
- Place sample 1 into a 500 mL round bottom flask.
- Add distilled water to the flask.
- Put the flask on a hot plate
- Connect a condenser column to the mouth of the flask. I do not have a proper ground joint condenser column, so I will improvise and just attach a stopperable water condenser.
- Connect the condenser to a water supply. I will use a tank of cold water and a pump.
- Turn the hotplate to its hottest setting and switch on light stirring.
- Wait for an hour.
- Switch off the hot plate and stir and wait for the setup to cool down.
- Switch off the condenser column's water supply.
- Set up a gravity filtration and wait for a clear liquid to pass through.
- Test the pH of the solution in the flask using pH strips.
- If the ph is even slightly acidic, add a few drops of universal indicator.
- Add the sodium hydroxide solution using a dropper until the pH is at 7 or 8.
- When this is done, transfer the solution to a round bottom flask and boil off most of the water. Do not put the stir bar in or the salts may crystallize around it.
- Test the nitrite and nitrate level using nitrite and nitrate test strips.
- Record the nitrite and nitrate concentration.
- Thoroughly clean all equipment that was used.
- Take sample two and place it in a 50 mL erlenmeyer flask.
- Bend two glass tubes, one to go from a flask generating steam in a flask containing carbon, and one to go from the flask containing carbon to a flask with water.
- Connect the first tube to a 500 mL flask filled with distilled water and a stir bar to prevent bumping.
- Clamp the carbon containing flask to a retort stand.
- Connect the two glass tubes into a size 3 stopper. Put the tube that will go to the steam generator deep into the stopper.
- Put the stopper in the flask so that the deeper tubing goes all the way to under the carbon.
- Connect a size 6 ½ stopper to the open end that will go to the steam generator.
- Place the other glass tube ending into a 250 mL boiling flask with water at the bottom.
- Set up an ice bath around the 250 mL boiling flask.
- Turn the hot plate to its hottest setting and switch on light stirring.
- When the water starts boiling, connect the 6 ½ stopper to the top of the 500 mL boiling flask.
- Add universal indicator into the 250 mL boiling flask.
- Wait for an hour and then switch off the hot plate. Remove the 250 mL boiling flask to avoid backflow.
- Add the dilute sodium hydroxide solution to the solution until the pH is neutral.
- Boil off most of the water, then test the nitrite and nitrate level.
- Record which method gave better results.
Part 3: Biological decomposition
- Repeat the adsorption steps from part 1 three times.
- Convert the NO2 to nitrite and nitrate using the method that worked best in the previous step.
- Once enough nitrite and nitrate salts have been collected and concentrated, they will be fed to a mixture of algae (Spirulina) and nitrobacter.
- An automatic light timer with an LED light will be set up to provide light to the algae and nitrobacter and the water temperature will be maintained 78 F.
- An optical density measurement device will be used to measure the algae growth. To measure optical density an arduino based system will be used, this was built as a side project. The system will take the optical density reading twice a day.
- Once a day, the nitrite and nitrate level will be measured.
- When the nitrate level drops back to what it was before, the experiment is over.
Materials
- Heat proof tube
- Thin PVC pipe
- 3 3 way adapters
- 3 male adapter joints
- 2 female adapter joints
- Valve
- 2 size 4 stoppers
- Snowblower
- Gas and oil for snowblower
- Arduino based NO2 sensor
- MicroSD card
- Computer
- Heavy duty epoxy resin
- 2 ladders
- Board
- Activated charcoal (granulated as powdered charcoal was difficult to use)
- Extension cord
- 500 mL boiling flask
- 250 mL boiling flask
- Distilled water
- Hot plate and stirrer.
- Stir bar
- Size 6 ½ stopper
- Size 3 stopper
- Filter and Filter paper
- 50 mL erlenmeyer flask
- Universal indicator and pH strips
- Dilute Sodium Hydroxide solution
- Retort stand
- Condenser
- Glass tubing
- Automatic light timer
- LED light bulb
- Arduino based optical density meter
- Algae culture media
- Spirulina algae.
- Nitrobacter in the form of fish tank nitrifying bacteria
- Nitrite and Nitrate test strips
Data List
For the adsorption part of the project, I will be taking a real time measurement of the treated NO2 concentration and one measurement of the untreated NO2 concentration. The treated gas data will then be documented. Every time this part is repeated, the data will be taken again. For the second part of the project, I will take two measurements, the nitrite and nitrate levels from the first method, and the nitrite and nitrate measurement from the second method. This will help me conclude which method is better. For the final part of the experiment, I will monitor the time that is taken for the nitrite and nitrate to be destroyed. This data will help judge the effectiveness of the project.
Risk assessment
Here are the risk associated with the project -
- Very dilute sodium hydroxide solution is used, however, goggles and latex gloves should be used.
- The fumes coming from the snowblower contain carbon monoxide and should not be inhaled.
- Epoxy is toxic and gloves should be worn before handling it
- While the acid produced will be extremely dilute, it is still necessary to wear goggles to avoid eye injury.
- The nitrobacter and algae were both purchased commercially and are not hazardous.
Bibliography
- Fang, M.-L., Chou, M.-S., Chang, C.-Y., Chang, H.-Y., Chen, C.-H., Hsieh, Y.-K., & Lin, S.-L. (2019) Retrieved from http://www.aaqr.org/files/article/9034/17_AAQR-19-09-OA-0439_2568-2575.pdf
- Nicholls , D. G., & Ferguson, S. J. (2013). Respiratory Chains. In Bioenergetics (Fourth Edition) 2013 (4th ed., pp. 91–157). Academic press. doi: https://doi.org/10.1016/C2010-0-64902-9
- Bothe, H., Ferguson, S., & Newton, W. E. (2006). Biology of the Nitrogen Cycle (1st ed.). Elsevier Science
- Kamino, Y., Yasuda, K., Inoue, S., & Onitsuka, S. (1972, November). Retrieved from https://www.jstage.jst.go.jp/article/jpi1959/14/2/14_2_147/_pdf
- (1999, November). Retrieved from https://www3.epa.gov/ttncatc1/dir1/fnoxdoc.pdf

