Prospects for Brain Computer Interfaces: Neuralink Versus Stentrode
Abstract:
Bibliography/Citations:
No additional citationsAdditional Project Information
Research Plan:
Research Question:
What are the advantages and disadvantages of the invasive Neuralink method and the non-invasive Stentrode method?
Hypothesis:
Adverse event data analysis of invasive and non-invasive Brain Computer Interfaces will show that the risk of the invasive approach is greater than the non-invasive.
Methods:
Procedures -
1. Conduct research in the field of BCI through reading primary (pre-clinical and clinical trials) and secondary literature
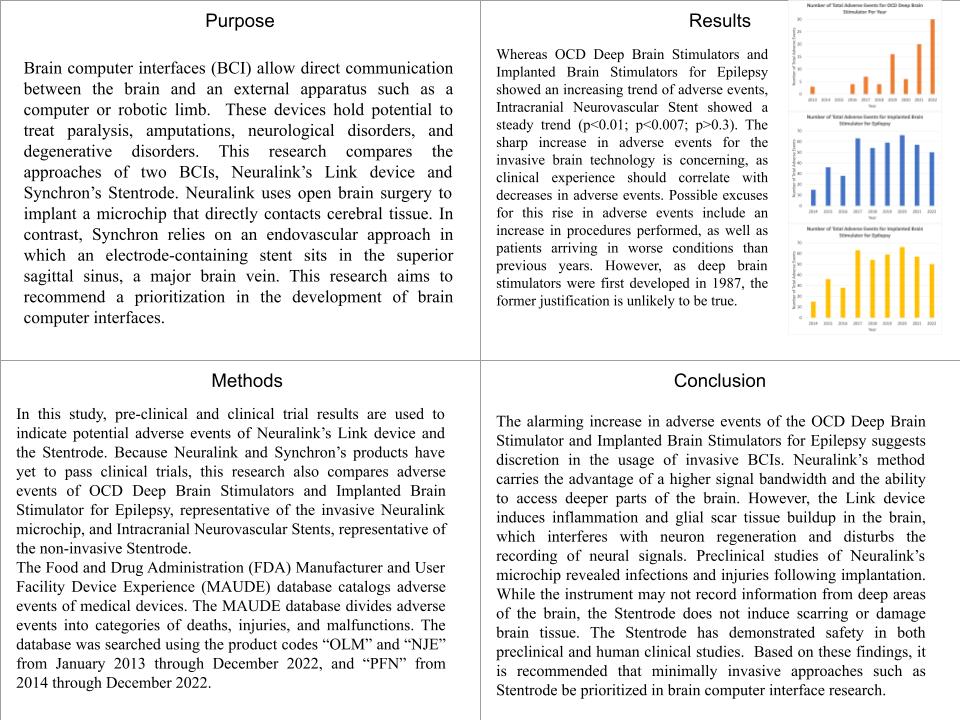
2. Collect monthly and annual data on total adverse events, deaths, injuries, and malfunctions of OCD Deep Brain Stimulator Adverse Events and Implanted Brain Stimulator for Epilepsy, representative of the invasive Neuralink, and Intracranial Neurovascular Stent, representative of the non-invasive Stentrode
Data Analysis -
Use bar graphs to visualize the results. Compare adverse event trends by comparing T-test results of 2013 / 2014 months and 2022 months. Use adverse event trends, as well as previous research, to draw conclusions about safety of similar products. Consider potential adverse events for implanted BCIs and endovascular BCIs.
Bibliography:
Fisher, Robert S. “Intravascular Stimulation of the Motor Cortex.” Nature Biomedical Engineering, vol. 2, no. 12, Dec. 2018, pp. 883–84. PubMed, https://doi.org/10.1038/s41551-018-0330-y.
Gardner, John. “A History of Deep Brain Stimulation: Technological Innovation and the Role of Clinical Assessment Tools.” Social Studies of Science, vol. 43, no. 5, Oct. 2013, pp. 707–28. PubMed Central, https://doi.org/10.1177/0306312713483678.
MAUDE - Manufacturer and User Facility Device Experience. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed 13 Mar. 2023.
Opie, Nicholas L., et al. “Focal Stimulation of the Sheep Motor Cortex with a Chronically Implanted Minimally Invasive Electrode Array Mounted on an Endovascular Stent.” Nature Biomedical Engineering, vol. 2, no. 12, Dec. 2018, pp. 907–14. www.nature.com, https://doi.org/10.1038/s41551-018-0321-z.
Raza, Samad A., et al. “Endovascular Neuromodulation: Safety Profile and Future Directions.” Frontiers in Neurology, vol. 11, Apr. 2020, p. 351. PubMed Central, https://doi.org/10.3389/fneur.2020.00351.
Questions and Answers
1. What was the major objective of your project and what was your plan to achieve it?
To compare the Neuralink microchip and the Stentrode and to recommend a prioritization in the development of brain computer interfaces.
a. Was that goal the result of any specific situation, experience, or problem you encountered?
After coming across a video of a monkey playing pong with its mind, I was extremely fascinated by Brain Computer Interfaces and wanted to dive deeper into the field.
b. Were you trying to solve a problem, answer a question, or test a hypothesis?
I wanted to answer the question of the advantages and disadvantages of invasive and non-invasive BCI.
2. What were the major tasks you had to perform in order to complete your project?
I searched the FDA MAUDE database and record in an Excel sheet the monthly adverse event data from 2013 to 2022 of three products.
3. What is new or novel about your project?
a. Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
Yes. I have not used the FDA MAUDE database nor completed data analysis on a scale as large as this before.
b. Is your project's objective, or the way you implemented it, different from anything you have seen?
Many neurological medical devices have been introduced to the market via fast-tracked approval processes by the FDA. This work is novel in that it examines adverse event data for these devices to compare adverse event data trends over the past decade.
c. If you believe your work to be unique in some way, what research have you done to confirm that it is?
The trends of adverse event data can be valuable for identifying areas where device redesign is needed.
4. What was the most challenging part of completing your project?
a. What problems did you encounter, and how did you overcome them?
The most challenging part was coming up with a project idea after doing research in the Brain Computer Interface field.
b. What did you learn from overcoming these problems?
I learned that with more and more research, ideas are naturally brainstormed.
5. If you were going to do this project again, are there any things you would you do differently the next time?
I would compare invasive and non-invasive BCIs in general, using products from companies other than Synchron and Neuralink as well.
6. Did working on this project give you any ideas for other projects?
This project proved to me my passion and interest in Brain Computer Interface! I would like to complete further anaylsis regarding the comparision between non-invasive and invasive BCI technologies. Another project idea is to look at device or patient problem classifications in the FDA MAUDE database to understand the root of the adverse events. In addition, I would like to further analyze and draw conclusions from the deaths, injuries, and malfunctions classifications data that I recorded.
7. How did COVID-19 affect the completion of your project?
The project was completed online, so Covid-19 had no effects.